Note: When requesting cIRB review, study teams must satisfy the requirements of each IRB and the funding agency or sponsor (as applicable), prior to initiating the research.
UNMC cIRB Initial Review Process
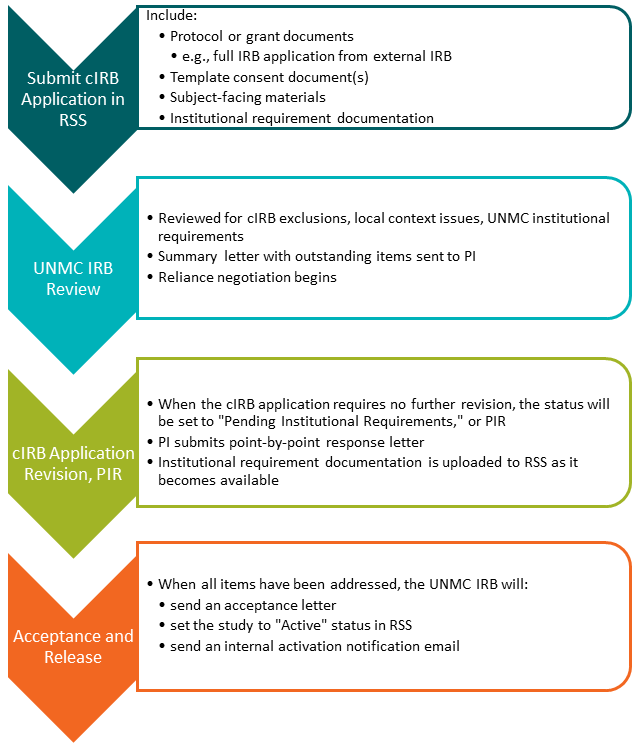
External cIRB Process
- Contact your external cIRB representative for details. Each IRB operates differently.
- IRB approval is needed prior to initiating research
Funding Agency/ Sponsor Process
- Contact your funding agency/sponsor representative for details. Each group operates differently.
- Funding agency/sponsor approval is needed prior to initiating research
Post-acceptance Study Activities
In general, the external IRB of record will be responsible for reviewing most study activities. In some cases, these may also need to be submitted to the UNMC IRB.
Things to submit to the external cIRB:
- Amendments
- Continuing Reviews
- Single-subject Deviation Requests
- Short-form Requests
- Incident Reports
- Adverse Event Reports
- Study Completion Reports
Note: In most cases, submit to the external IRB. If determined to be necessary, the external cIRB will notify the UNMC IRB directly or request the study team notify the UNMC IRB.
Things to submit to the UNMC IRB:
- New or modified Conflicts of Interest, management plans or additional external cIRB requirements
- Copies of reports made to OHRP and/or FDA
- Copies of Incident Reports submitted to the cIRB
- Copies of Internal Adverse Event Reports submitted to the cIRB
- Personnel Changes
- Note: personnel are NOT permitted to work on a study until UNMC IRB approval is received.
- Annual Status Update via Demographic Recruiting Numbers Form
- Study Closure Notifications
- Note: Send a notification in the message portal, indicating the study is now closed. Upload applicable documentation.


