The purpose of the College of Dentistry's bioregulation research is to understand and manipulate the immune system, inflammation, and bone physiology to lead to more effective treatment of numerous conditions, including infectious diseases, chronic viral infection, chronic inflammatory diseases (arthritis, endodontic infection, and periodontitis), autoimmune diseases (multiple sclerosis and insulin-dependent diabetes), metabolic bone diseases (osteoporosis), abnormal craniofacial development and cancer.
Cell surface receptors linked to cytosolic cell signaling systems activate transcription factors for expression of secreted cytokines of immune, inflammatory and bone cells in order to regulate the immune system, inflammation, bone-turnover and tissue repair.
To understand cytokine secretion important for regulation of biological processes, the BRG examines receptors, cell signaling pathways, genes, promoter sequences, and associated transcription factors. The BRG is also involved in discovery of novel compounds that are natural or synthetic that antagonize or agonize these pathways and, thus, more predictably could be used to improve inflammatory, immune and bone responses.
The research endeavors of the Bioregulation Research Group (BRG) address several human disease problems:
- Beneficial bone remodeling caused by controlled tooth movement (orthodontics)
- Alveolar bone resorption (periodontitis), dental pulp necrosis (endodontics), and neurological dysfunction (multiple sclerosis) brought about by chronic inflammation, dysfunctional immune responses, or exhausted immune responses during persistent bacterial, fungal, or viral infections.
- Immune modulating virulence factors produced by microbes that could cause chronic inflammation, persistent infection, and excessive bone resorption. These factors include lipopolysaccharide and other pathogen-associated molecules derived from bacteria; farnesol, a virulence factor derived from the fungus Candida albicans; and L- and L* protein produced by the Cardioviruses.
In vitro protocols used by the BRG involve:
- Cytokines: While an understanding of cytokine production can be obtained using harvested tissues and exudates from specific microenvironments, a mechanistic understanding of regulation and modulation of these cytokines can be done in vitro using immune, inflammatory, and bone cells in culture.
- Pharmaceuticals that can modulate these cytokines are first tested in vitro. These pharmaceuticals include resveratrol, a phytoestrogen derived from plant foods such as red grapes, non-antibacterial tetracyclines, such as subantimicrobial doxycycline (which is approved for human use and has been tested in human clinical trials at the UNMC College of Dentistry), and the cholesterol-lowering drug, simvastatin, which has novel bone-augmenting properties.
In vivo rodent model protocols used by the BRG include:
- Theiler’s murine encephalomyelitis virus (TMEV) causes a chronic viral infection that leads to neuroinflammatory disease similar to Multiple Sclerosis in some strains of mice. We investigate innate immune system factors responsible for chronic TMEV infections in susceptible mice and compare these responses to resistant mice.
- Experimental autoimmune encephalomyelitis (EAE) in mice is another model of human Multiple Sclerosis. We bring about EAE by injecting myelin proteins derived from the white matter of the central nervous system into mice. We evaluate anti-inflammatory agents (e.g., resveratrol) on neurological dysfunction brought about by chronic viral infection or EAE.
- Experimental bone growth in the bilateral rat mandible can measure excessive bone resorption/remodeling that can occur due to chronic microbial infection near bone, such as periodontal disease (periodontitis). We examine the effects of topical drugs (e.g., simvastatin) on bone growth in a bilateral rat mandible model of periodontitis.
Induction of cytokines during the innate immune response to persistent viruses
 All humans are infected with viruses throughout their lifetime. While most of these virus infections stimulate innate and adaptive immunity and are eliminated, it is estimated that each human is infected by 8-10 viruses that persistently stimulate the immune systems and are never eliminated. It is hypothesized that in some cases these persistent viral infections lead to diseases, such as Multiple Sclerosis (MS). Dr. Petro has had funding from the National Multiple Sclerosis Society, the Nebraska Center for Virology, and the Nebraska Center for Cell Signaling to investigate a mouse model of human MS in which a persistent viral infection in mice triggers symptoms similar to MS in humans. As a model for persistent viral infections, his laboratory is focusing upon the innate immune response to the Theiler's Murine Encephalomyelitis Virus (TMEV). In some strains of mice TMEV persistently infects macrophages of the immune system but is never eliminated within the lifetime of the individual mouse. In other strains of mice, TMEV is eliminated within a few weeks. As a result, susceptible mice with persistent TMEV infections end up with a neuroinflammatory disease similar to human MS. Dr. Petro has seen that failures within the innate immune system are causes of TMEV persistence. The innate immune response to viral infection depends on Interferon Response Factor-3 (IRF-3), Type I interferons (IFN-alpha/beta), IL-12, and IFN-gamma.
All humans are infected with viruses throughout their lifetime. While most of these virus infections stimulate innate and adaptive immunity and are eliminated, it is estimated that each human is infected by 8-10 viruses that persistently stimulate the immune systems and are never eliminated. It is hypothesized that in some cases these persistent viral infections lead to diseases, such as Multiple Sclerosis (MS). Dr. Petro has had funding from the National Multiple Sclerosis Society, the Nebraska Center for Virology, and the Nebraska Center for Cell Signaling to investigate a mouse model of human MS in which a persistent viral infection in mice triggers symptoms similar to MS in humans. As a model for persistent viral infections, his laboratory is focusing upon the innate immune response to the Theiler's Murine Encephalomyelitis Virus (TMEV). In some strains of mice TMEV persistently infects macrophages of the immune system but is never eliminated within the lifetime of the individual mouse. In other strains of mice, TMEV is eliminated within a few weeks. As a result, susceptible mice with persistent TMEV infections end up with a neuroinflammatory disease similar to human MS. Dr. Petro has seen that failures within the innate immune system are causes of TMEV persistence. The innate immune response to viral infection depends on Interferon Response Factor-3 (IRF-3), Type I interferons (IFN-alpha/beta), IL-12, and IFN-gamma.
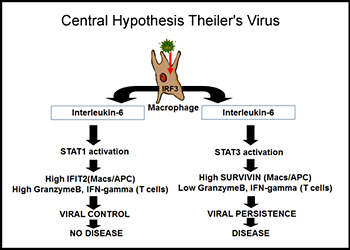
Other cytokines that are induced, such as IL-23, do not cause virus elimination but likely promote MS-like disease. In addition to innate cytokine production, apoptosis (programmed cell death) is triggered in virus-infected cells. Apoptosis prevents viral persistence and infection into surrounding cells. Induction of Type I interferons, repression of IL-12, and apoptosis are all dependent on a transcription factor called interferon regulatory factor 3 (IRF-3). Dr. Petro has found that IRF-3 of mice that cannot eliminate TMEV differs from IRF-3 of mice that can control this virus. Similar differences in IRF-3 are found in human populations. Therefore, interferon, IL-12, and apoptosis are altered in macrophages from susceptible mice infected with TMEV. Interestingly, TMEV and many viruses like it encode a protein, termed L-protein, that interacts with IRF-3 and modulates its activity and another protein L*-protein that interferes with apoptosis. The central hypothesis of this line of research in Dr. Petro’s lab is that differences in host IRF-3 or viral proteins that modulate IRF-3 underlie persistent infections with certain viruses.
Cytokine Expression
Persistent microbial infections lead to pathology in host tissue because of persistent inappropriate cytokines produced by the inflammatory and immune systems. Therefore, cytokines produced by cells of the bone microenvironment are being examined in in vitro and in vivo bone turnover systems to define more clearly their bone resorptive effects during chronic gingival infection. To gain an insight into the finer details of cytokine induction pathways, promoter constructs of inflammatory cytokine genes are being transfected into primary cells and cell lines from the bone microenvironment to study mechanisms of gene activation and cytokine production. In particular, the influence of bacterial virulence factors, estrogen levels, and new pharmaceutical candidates are being tested using these promoter constructs of inflammatory cytokine genes. Long-term goals include defining gene signaling factors which could be manipulated to direct cytokine production to yield net bone formation. Stem cell lines from individuals could be developed to reseed periodontal defects and stimulate bone regeneration.
Regulation of Cytokine Production
It is quite clear that CD4 T cell and APC cytokine production is induced by appropriate ligation of cell membrane proteins, transmembrane signaling cascades, and activation of cytokine-specific transcriptional factors interacting with promoters of cytokine genes. However, excessive cytokine induction occurs when inflammatory and immune responses are stimulated by persistent microbial infection. The Bioregulation Group is committed to elucidation of cytokine induction pathways and discovery of counter-measures that control cytokine expression. Dr. Petro is using a plant compound called resveratrol which has been shown to be anti-inflammatory and which controls immune responses. The central hypothesis here is that resveratrol mimicking of internal cell signaling and transcriptional mechanisms controls inflammation and immune responses. In the end, resveratrol induces counteracting cytokines or factors that dampen inflammatory and immune responses. New information about resveratrol could be used to promote protective immune responses so that infecting microbes are eliminated or controlled, chronic inflammation is prevented, and immune responses do not become exhausted. In order to achieve these goals, Dr. Petro is using this unique compound as a therapy in experimental model systems to manipulate signaling and transcriptional pathways.
Experimental Bone Turnover
The periodontium (gum, mucosa and underlying bone) provides a unique in vivo opportunity to study the sequence of inflammatory cell cytokine production and immunoregulation following tooth movement, bacterial insult, or wounding. Movement of the tooth within the periodontal ligament space, introduction of bacterial products, or bone regenerative procedures result in an influx of inflammatory cells leading to increased local levels of cytokines and markers of bone turnover. These products can be sampled from specific microenvironments with special devices and analyzed (ELISA). Such approaches allow identification of protocols where local or systemic drug interventions would be most effective.
Chronic Inflammation
Chronic bacterial insult, modulated by genetic and other environmental factors, is the hallmark of inflammatory periodontal disease with alveolar bone destruction. This disease also provides an accessible source of inflammatory cells and products to study the nature of chronic inflammation. BRG investigators are probing cytokine/inflammatory mediator profiles and mechanisms of production in gingival exudate and exudate from specific microenvironments, and in cell extraction/culture. By understanding the association of cytokines with periodontal inflammation and bone destruction, the use of contradictory cytokines or cytokine antagonists will be possible for alveolar bone loss prevention and regeneration. Anti-inflammatory therapy, inhibition of host-derived tissue-destructive matrix metalloproteinases, and local delivery of antibiotics also are being studied.
The research at the UNMC College of Dentistry is composed of the Cruzan Center for Dental Research (CCDR), the Bioregulation Research Group (BRG), the BioMaterials Research Group (BMRG), and the Nebraska Center for Cell Signaling (NCCS). The college has nine modern basic science research laboratories and four large research operatories that are well-equipped for state-of-the-art basic and translational research in cell signaling in cancer, biomaterials, and regulation of inflammation and bone turnover. The college has a highly-skilled faculty of basic and clinical science scholars who have strong collaborations with other Colleges at UNMC as well as universities throughout the nation and world.
Clinical Operatories
The Cruzan Center for Dental Research (CCDR) is the home of the College of Dentistry (COD) clinical research program. To facilitate the translation of basic science research to clinical application, the CCDR has a clinical research center with 4 large research operatories, a reception area, a secure data entry/storage room, preparation laboratory, and supporting staff offices. Dental operatories are available for procurement of human clinical samples and measurements, as well as performance of clinical trials. The operatories are each approximately 150 square feet and contain a full complement of dental equipment appropriate for clinical procedures.
Basic Science Laboratories
The basic science labs are focused upon understanding craniofacial biomaterials, as well as cell signaling pathways of DNA damage responses, of cell adhesion molecules, and of inflammatory and immune responses. To facilitate the basic research the COD has a basic research center with 9 secure research labs with supporting staff offices and newly renovated animal care facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All of the labs have the newest molecular and cell biology equipment, which include a live cell microscopy suite, fluorescent activated cell analyzer, real-time PCR analyzer, and infrared imaging system.
Research Team
Larry D. Crouch | Jeffrey B. Payne | Thomas M. Petro | Richard A. Reinhardt
Student Involvement
UNMC College of Dentistry students are involved in bioregulation research projects through the Ameritas Nebraska Dental Student Research Group.
Collaborations
- University of Texas Health Science Center, San Antonio Dental School
- University of Nebraska-Lincoln School of Biological Sciences
- UNMC Eppley Institute and Lied Transplant Center
- The State University of New York - Stony Brook, School of Dental Medicine
- University of Florida College of Dentistry
- Nebraska Center for Virology
- University of Oklahoma Health Sciences Center College of Public Health
- UNMC College of Medicine
- University of Helsinki Institute of Dentistry

