Sidharth Mahapatra Lab

As a pediatric intensivist working in an area with an exceptionally high incidence of pediatric brain tumors, I am concentrating my strengths in molecular neurobiology and translational science to investigate pediatric medulloblastomas (MB), the most common malignant brain tumor of childhood. With an annual incidence of 0.4 per 100,000, Nebraska ranks in the top 10 states for this devastating tumor. With the mainstay of therapy being surgical resection followed by radio- and chemotherapy, we routinely care for these patients at UNMC.
MBs are divided into 4 primary subgroups, i.e. Wingless (WNT), Sonic-hedgehog (SHH), group 3, and group 4. Patients with WNT, SHH, and group 4 tumors experience better survival odds and quality of life than those with group 3 tumors, who have a 5-year overall survival <50%. Poor insight into pathophysiology coupled with high metastases at diagnosis and recurrence rates bestow dismal outcomes to patients with group 3 tumors.
To understand group 3’s elusive pathophysiology, I spent my early investigator career delving into the significance of tumor suppressor genes on chromosome 17p, a locus sustaining frequent mutations in a majority of MB tumors. My lab has identified several tumor-suppressive micro RNAs, their oncogenic targets, and deregulated pathways that may confer an aggressive phenotype to group 3 tumors. I am now investigating how to capitalize on this mechanistic insight to generate safe, well-tolerated, targeted therapeutic approaches that may mitigate the need for high doses of radiation and chemotherapy, with the hope of improving not only survival but also quality of life for patients with group 3 MB.
Current Members
Sidharth Mahapatra, MD, PhD
Associate Professor, UNMC Department of Pediatrics
Associate Professor, UNMC Department of Biochemistry and Molecular Biology
Chair, Nebraska Children’s Brain Tumor Collaborative
Co-Director, Pediatric Cancer Research Group

Ranjana Kanchan, PhD
Post-doctoral Associate 2016-2020
Instructor 2020 - present

David Doss
MD, PhD student 2021-2024

Maria Burkovetskya , MS
Research Technologist II 2024 - present

Abhishek Bhattacharya, PhD
Post-doctoral associate 2024 - present
Former Members
Naveenkumar Perumal, PhD
Instructor 2017-2022

Prakadeeswari Gopalakrishnan, PhD
Post-doctorate associate 2022-2023

1. Tumor suppressive microRNAs in medulloblastoma
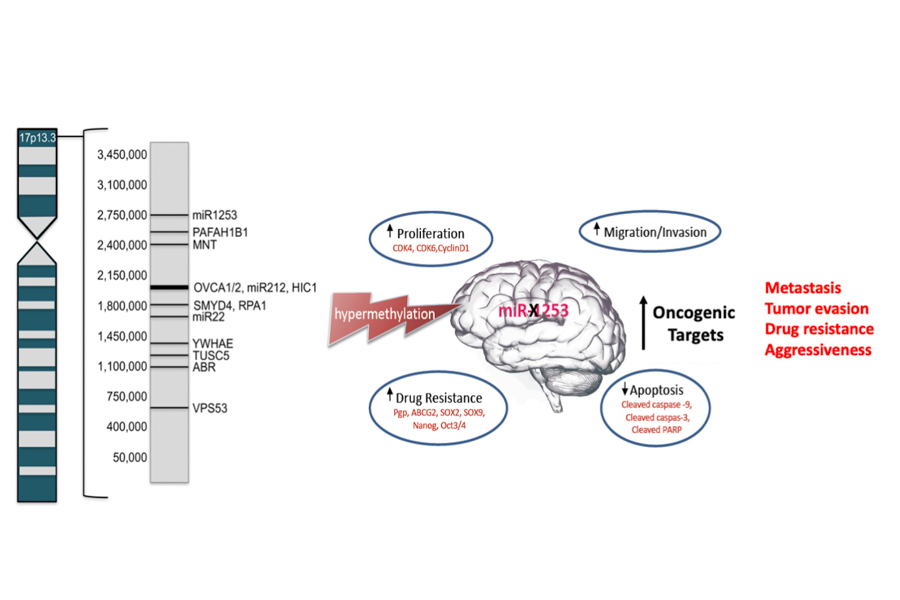
Haploinsufficiency of 17p13.3 has been reported in up to 50% of MB cases and correlated with poor prognosis. Chromosomal mapping and microdeletion studies of 17p13.3 have revealed many putative tumor suppressor genes on this locus, including several microRNAs. MicroRNAs (miRs) regulate gene expression by binding to the 3’- untranslated region (3’-UTR) of target mRNAs and inhibiting their expression. MiRs can serve as ideal tumor suppressor genes, given their capacity to serve a regulatory role for hundreds of target proteins.
MiR-1253, found on the terminal end of 17p13.3, is a brain enriched miR that plays a critical role in cerebellar development. We showed that miR-1253 possesses strong tumor-suppressive properties in medulloblastoma and is silenced in MB tumors by hypermethylation. MiR-1253 suppresses the expression of several key oncoproteins, including cell cycle checkpoint proteins ( CDK4, CDK6, cyclin D1), mitochondrial iron transporters (ABCB6, ABCB7, ABCB8), and the immune checkpoint regulator CD276/B7-H3.
2. Elevated iron transport and medulloblastoma aggressiveness
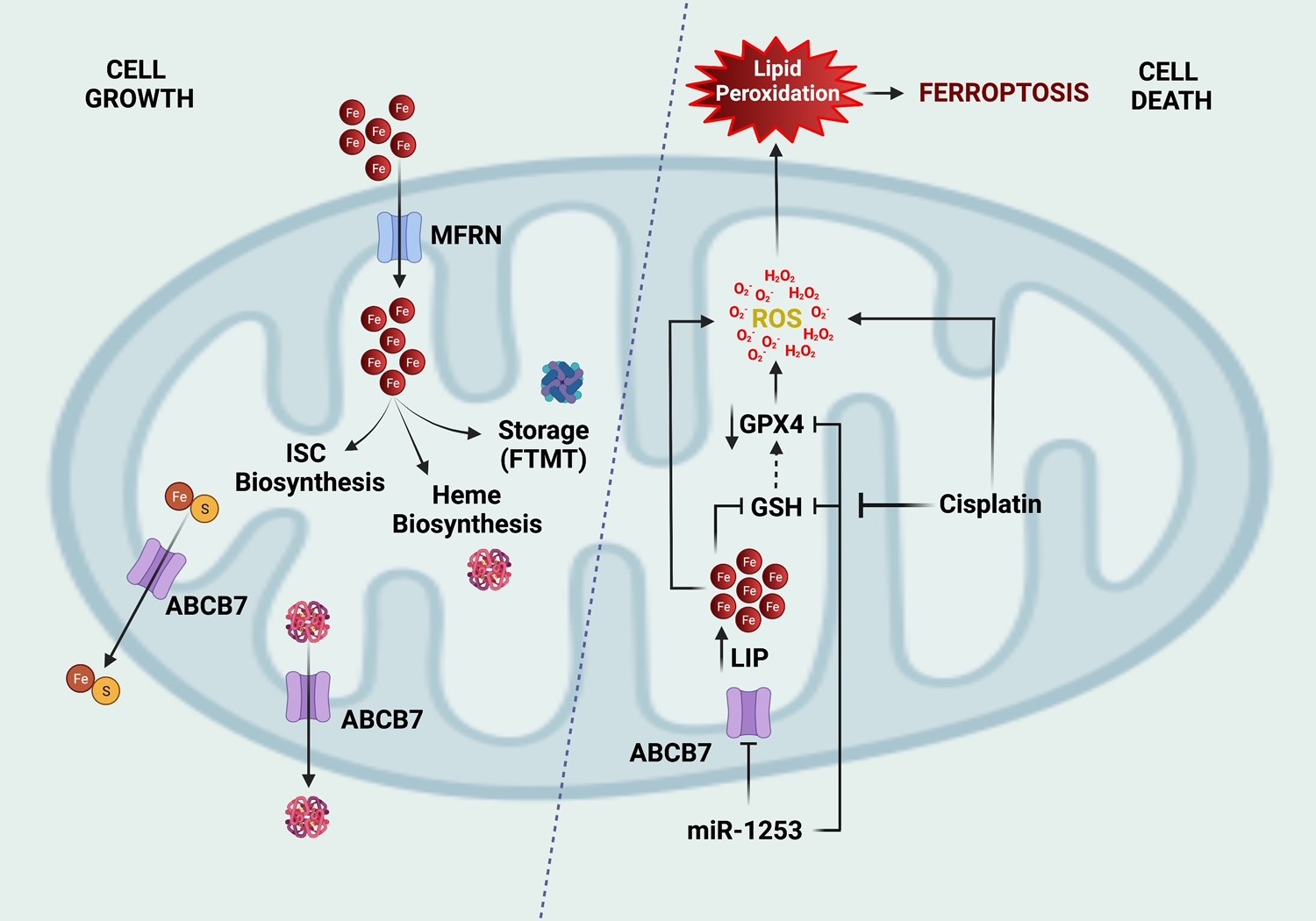
Rapidly dividing cancer cells require iron as a critical substrate. Mitochondrial ABC transporters are intimately involved in supplying iron-sulfur and heme proteins to buttress the elevated need for proteins essential to cancer cell growth and proliferation. ABCB7 is expressed in the inner mitochondrial membrane and involved in iron-sulfur cluster export from the mitochondria after synthesis. It is also enriched in group 3 MB and inhibited by miR-1253. Repression of ABCB7 triggers iron overload, ROS generation, and lipid peroxidation, culminating in cell death by ferroptosis. Moreover, the cytotoxic actions of cisplatin, partially mediated by ferroptosis, are potentiated by ABCB7 repression in group 3 tumors. These novel findings unlock the potential to exploit the high iron needs of medulloblastomas as a targetable vulnerability to enhance the effect of chemotherapy in aggressive MB tumors.
3. Targeting B7-H3 in group 3 medulloblastoma
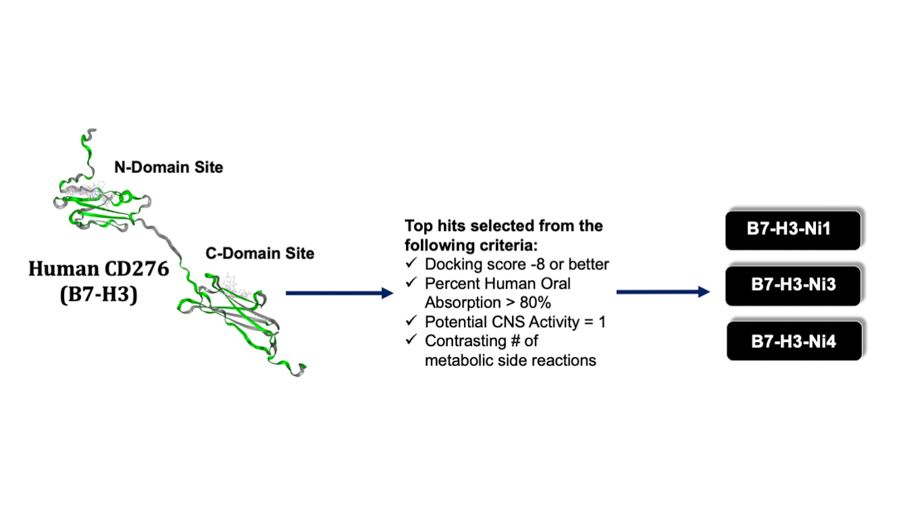
B7-H3 is an immunomodulatory protein whose deregulation in many malignancies is linked to more aggressive, metastatic tumors, higher relapse rates, and drug resistance. B7-H3 can serve as an ideal targetable oncoprotein in MB for several reasons. First, similar to various other cancers, its expression is enriched in MB. Second, deregulated B7-H3 expression is strongly associated with poorer outcomes, especially in group 3 tumors. Third, B7-H3 inhibition attenuates tumor growth and mortality. We further showed that targeting B7-H3 in vitro resulted in appreciable inhibition of cancer cell migration, invasion, and the metastasis-associated protein, SLUG. These observations suggest that targeting the tumor-promoting properties of B7-H3 is an efficacious way to curb the aggressiveness of group 3 MB tumors. We are now exploring small-molecule inhibitors of B7-H3, generated through a structure-based drug discovery pipeline.
4. Transcriptome-driven drug discovery platform for medulloblastoma
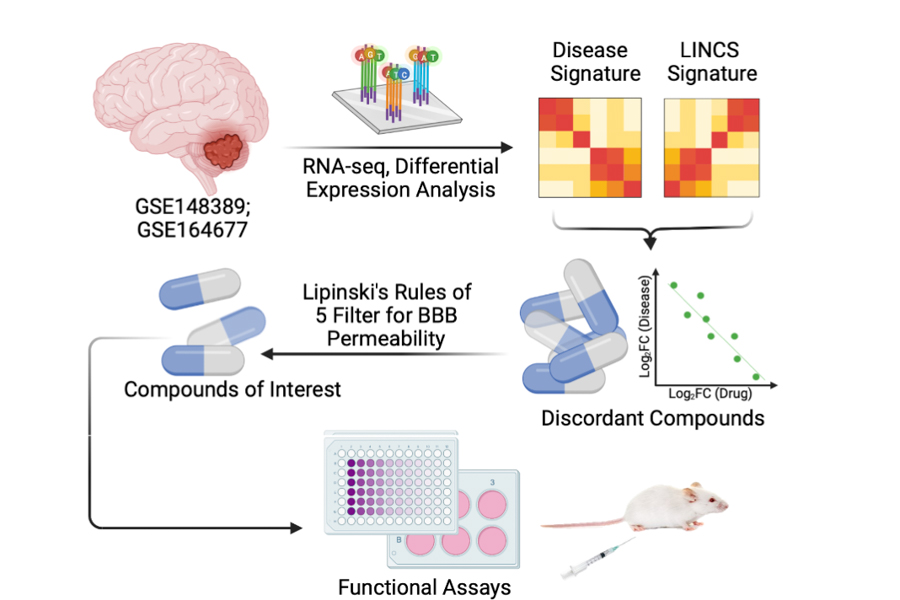
Medulloblastomas affect children with a 10-fold higher incidence than adults. MB therapy has largely depended on chemo-radiation regimens that result in appreciable toxicities, sometimes precluding effective dosing. Studying the tumor transcriptome may hold the key to designing better treatment option for children with MB.
In this study, we approached novel drug discovery for MB by comparing the tumor transcriptomes of 2 cohorts of pediatric MB patients against a library of integrated network-based cellular signatures (LINCS), which houses omics signatures for cellular perturbations to known drugs. Our intent is to isolate those drugs capable of reverting the tumor transcriptome to a more normal cerebellar phenotype. We applied 2 additional filters, including FDA approval in pediatric patients and blood-brain barrier permeability, and have isolated several promising compounds with strong anti-neoplastic properties in group 3 MB. Amongst the most promising drug classes are statins, selective serotonin-reuptake inhibitors (SSRIs), and tricyclic antidepressants (TCAs).
Funding
- Project 1: Deep Investigation of Gene Silencing on 17p13.3 Identifies Targetable Vulnerabilities Informing Novel Therapeutic Strategies for Pediatric Medulloblastomas
- Project 2: Using Connectivity Mapping to Identify Anti-Neoplastic Agents for High-risk Medulloblastoma
- Project 3: Developing a Regional Medulloblastoma Core Facility at the University of Nebraska Medical Center
Project Title: Elucidating mitochondrial iron transporter-mediated ferroptosis in group 3 medulloblastoma
Project Title: Targeting B7-H3 (CD276) mitigates tumor aggressiveness in group 3 medulloblastoma