Diagnostics & Therapeutics
Iwen Lab
Peter C. Iwen, MS, PhD, D(ABMM), F(AAM)
Professor, UNMC Department of Pathology, Microbiology and Immunology
Senior Biosafety Officer, UNMC / UNO
Director, Nebraska Public Health Lab

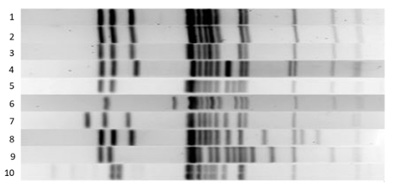
Figure. Salmonella serotype DNA fingerprints generated by pulsed field gel electrophoresis. Each lane denotes a separate isolate of Salmonella with lanes 1-3 showing indistinguishable fingerprint patterns when compared to the other lanes. Following a investigation, these 3 related strains were found to represent a clonal spread of one Salmonella strain associated with an outbreak of salmonellosis.
Research activities in the lab of Peter C. Iwen, PhD, include the development and optimization of molecular assays for the identification of microbial pathogens with an emphasis on fungi and bacteria (including the Mycobacterium species).
Original work in this laboratory showed that the internal transcribe spacer region within the rDNA complex was a useful target for the identification of both the common and uncommon fungal and mycobacterial species.
Subsequent work has been in utilization of whole genome sequencing in collaboration with bioinformaticians to analyze microbial pathogens to investigate foodborne and nosocomial outbreaks as well as to monitor for genetic targets to identify the presence antimicrobial resistance.
Wang Lab

The laboratory of Guangshun (Gus) Wang, PhD, focuses on the identification, characterization and engineering of novel antimicrobial agents based on structural, bioinformatics, and functional studies. Our ultimate goal is to develop novel compounds that curb pathogenic microbes, especially difficult-to-kill microbes such as methicillin-resistant staphylococcus aureus, also known as MRSA.
Naturally occurring antimicrobial peptides are universal effector molecules that directly eliminate invading pathogenic bacteria, fungi, viruses, and parasites. In mammals, including humans, such peptides may also modulate the adaptive immune systems. To date, more than 2,890 antimicrobial peptides have been identified in bacteria, fungi, plants and animals.
To better manage this information, my laboratory has established the Antimicrobial Peptide database as a tool for naming, classification, search, statistical analysis, prediction and design. Our database is also a useful resource for developing novel antimicrobial agents. These miniature proteins are capable of adopting a variety of three-dimensional structures, inspiring our design of natural mimics that benefit mankind. The objectives of our current NIH-funded projects are to develop antimicrobial peptides into novel antimicrobial agents, including covalent immobilization on medical implants.
We are particularly interested in an in-depth understanding of the functional roles of human antimicrobial peptides and their relationships with human diseases, including cancer. Recently, we have solved high-quality structures of human cathelicidin LL-37 and its important fragments by multidimensional nuclear magnetic resonance spectroscopy. Dioctanoyl phosphatidylglycerol (D8PG) has been established as a new and unique membrane-mimetic model, which enables the detection of Phe-PG and Arg-PG interactions. Based on three-dimensional structures, we have identified the most potent region within LL-37 against MRSA, thereby identifying a useful template for designing novel therapeutic compounds against this superbug (US Patent 7,465,784). To elucidate the mechanism of action, we are utilizing a variety of biophysical and biochemical techniques.
Our studies will lay the foundation for peptide engineering with a goal to overcome the hurdles (stability, toxicity, and production) on the way to the development of natural antimicrobial peptides into novel therapeutics.
Another research direction of high interest to us is to engineer molecules that control protein-mediated signal transduction pathways essential for bacterial survival or infection in collaboration with colleagues in the Center for Staphylococcal Research. The lead compound will be optimized by combining nuclear-magnetic-resonance-based library screening with rational design based on three-dimensional structures of protein-protein complexes.