Thomas Laboratory
Bacterial Pathogen Adaptations

Research projects in the laboratory of Dr Thomas focus on bacterial pathogen adaptations during stress and infection. Current projects aim to understand how Staphylococcus aureus and Staphylococcus epidermidis alter metabolism and energetics to optimize fitness during stress. Investigators in the Thomas laboratory routinely employ genetic, biochemical, and metabolomic approaches to dissect relevant questions. This research's long-term goal is to identify metabolic pathways or cellular targets of therapeutic importance that limit the pathogen's ability to adapt during infection.
Identifying molecular mechanisms that contribute to staphylococcal weak acid resistance
Staphylococci commonly encounter biological weak acids like lactate and acetate when residing on the human skin. While lactate is abundant in human sweat gland secretions, acetate is generally produced as a byproduct of staphylococcal carbon catabolism. Both lactate and acetate can inhibit bacterial growth and affect its survival under human sweat's low pH conditions. Yet, staphylococci thrive under these conditions and colonize human skin. Thus, we are interested in determining metabolic and physiological adaptations that enhance staphylococcal resistance to weak acid stress.
Weak acids like acetate typically exist in an anionic form under neutral pH. However, they combine with protons and enter cells when the external pH is close to the pKa of the weak acid. For instance, when the external pH is 4.8, 50% of the acetate will be protonated and can diffuse into the cytoplasm due to its neutral charge. Weak acid toxicity could arise due to two reasons—the first results from intracellular acidification. The acetic acid that enters the cell dissociates in the relatively neutral cytoplasm, releasing protons that contribute to acidification. The second reason for toxicity could result from anion accumulation. Acetate anions can significantly accumulate in the cytoplasm resulting in unknown physiological consequences. Current projects on this topic aim to identify cellular determinants of staphylococci that reduce intracellular acidification and deter the effects of anion toxicity.
Bacterial programmed cell death as a mechanism to maintain fitness under stress
Bacterial pathogens like staphylococci can efficiently transition between active growth and stationary phase in response to perturbations in nutrients and environmental stressors. The molecular changes that accompany their entry into stationary phase following stress are optimized for long-term survival. These changes enhance the pathogens' persistence and make them tolerant to various stressors, including antibiotics and the host immune system. However, the fate of stationary phase bacterial cells that undergo significant cellular damage due to stress and the contribution of these damaged cells to the overall population fitness remains unclear.
Our recent forays into this project primarily focus on testing the hypothesis that damaged cells are detrimental to overall population fitness and activate a cell death program that leads to their elimination. Their removal would allow competition for limiting nutrients to be confined to healthy cells and ensure that the surviving population is fit for re-entry into the growth cycle under favorable conditions. Additionally, we are in the process of identifying and characterizing proteins that play a role in bacterial PCD.
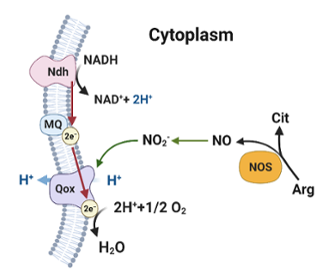
The importance of arginine catabolic pathways in adaptation to stress
Arginine catabolism in staphylococci occurs through three metabolic routes catalyzed by the enzymes: arginine deiminase, nitric oxide synthase and arginase. A major focus of our laboratory is to understand how these enzymes contribute to fitness during regular growth and when they are subject to various stress factors. Our laboratory is also interested in how these catabolic routes are regulated when cells undergo acidic, oxidative and nitrosative stress.
We have recently elucidated the importance of nitric oxide synthase in staphylococcal physiology. Host nitric oxide is a critical innate immune effector produced by mammalian nitric oxide synthase that targets bacterial pathogens. Thus, it was initially surprising that staphylococci would also encode nitric oxide synthase. Our work demonstrated that endogenous nitric oxide produced by staphylococci does not accumulate to toxic levels because it gets rapidly oxidized to nitrite, which stimulates respiration and growth. Current areas of research on this topic are focused on how nitrite derived from nitric oxide synthase stimulates staphylococcal respiration.
Lab Personnel
- Vinai Thomas, PhD (PI)
- Ryan Singh, PhD student
- Dhananjay Shinde, PhD