Coxiella Research
Gilk Lab

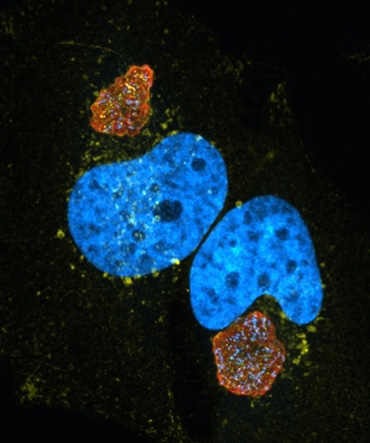
HeLa cells infected with Coxiella (red) and stained for the late endosomal marker CD63 (yellow) and host cell nucleus (blue - DAPI). Note the large size of the Coxiella containing vacuole.
Studying the Cause of Q Fever Endocarditis
Coxiella burnetii causes Q fever endocarditis, a disease that requires 18-24 months of antibiotic treatment and lacks an FDA-approved vaccine.
The goal of our research is to understand how Coxiella survives in the host cell, and identify new therapeutic targets.
During infection, Coxiella infects macrophages and survives in a unique, lysosomal-like compartment called the Coxiella containing vacuole. Formation of the Coxiella containing vacuole is essential for bacterial survival, yet we know little about its biogenesis and maintenance.
We are especially interested in how cholesterol and other lipids contribute to its formation and unique properties.
How Does Coxiella Modulate Host Cholesterol Levels?
We utilize a combination of cell biology, biochemistry, and molecular biology techniques to address the role of cholesterol in Coxiella-host cell interactions. Using a novel cholesterol-free tissue culture system, we discovered Coxiella does not require cholesterol for formation and growth of Coxiella containing vacuole, and is in fact sensitive to high levels of cholesterol. In addition to understanding why cholesterol is toxic to Coxiella, we are determining how Coxiella regulates host cholesterol levels.
Our studies suggest that cholesterol metabolism is an integral component of Coxiella pathogenesis, and therefor may be a viable drug target.
One potential mechanism to lower Coxiella containing vacuole cholesterol levels is bacterial-mediated transfer of cholesterol to another membrane. We found that Coxiella recruits the host cholesterol binding protein ORP1L to form membrane contact sites between the PV and host endoplasmic reticulum.
Using a combination of quantitative microscopy, live cell imaging, proteomics, and CRISPR technology, we are characterizing the structure and function of CCV-ER membrane contact sites.
We hypothesize that Coxiella enzymatically modifies host cholesterol by expressing two sterol-modifying enzymes, CBU1158 and CBU1206. These bacterial proteins may alter and decrease Coxiella containing vacuole cholesterol.
Using sterol profiling, we discovered that the lack of CBU1206 leads to elevated levels of the host oxysterol 25-hydroxycholesterol (25-HC), a potent regulator of cholesterol metabolism. We are further investigating the role of 25-HC during Coxiella infection, as well as characterizing sterol reductase mutants and enzymatic activity.
How Does Coxiella Regulate CCV pH?
While the Coxiella containing vacuole is a lysosome-like vacuole, it actively regulates CCV in a range that supports bacterial metabolism but decreases the activity of harmful host proteases which are designed to kill microbes.
Further, elevated CCV cholesterol leads to increased CCV acidification and bacterial death. In order to regulate CCV pH, we demonstrated that the bacteria actively blocks endosomal maturation, thus decreasing host lysosomes and expression of lysosomal proteases. We are using quantitative microscopy, live cell imaging, and CRISPR technology to decipher the molecular mechanism used by Coxiella to manipulate host cell lysosomes.
Modulation of Host Innate Immune Signaling
During bacterial infection, macrophages secrete chemokines, which recruit neutrophils to help control the infection. Using RNAseq, we discovered that Coxiella blocks IL-17 signaling by macrophages, thus decreasing chemokine secretion. Using a combination of genetic approaches (CRISPR, bacterial transposon mutants), immunoblotting, and chemokine analysis, we are elucidating the key host and bacterial proteins involved in IL-17 signaling during Coxiella infection.
Lab Members
- Stacey Gilk, PhD, associate professor
- Sushmita Adikhari, PhD student
- Rajendra Angara, PhD, postdoctoral associate
- Emily Heaton, PhD student
- Brigham Killips, PhD student
- Charles Porto, master's student
- Arif Sadi, PhD student
- Maggie Sladek, PhD student
- Laura Tellez Ramirez, research technologist
- Peyton VanWinkle, PhD, postdoctoral associate