Tumor Microenvironment Laboratory
Singh Laboratory
Rakesh K. Singh, PhD
Samuel M. and Janet L. Cohen Distinguished Professor in Pathology and Microbiology
Professor, UNMC Department of Pathology, Microbiology and Immunology
Vice Chair of Graduate Education, UNMC Department of Pathology, Microbiology and Immunology
Director, UNMC Immunology, Pathology, and Infectious Disease Graduate Program

Using human tumors xenografted in nude mice and murine tumor models, these studies have demonstrated the tumor microenvironment regulates angiogenesis, immunosuppression, progression, and metastasis. Further characterization of the cellular and molecular mechanisms underlying these processes is ongoing in our laboratory.
In addition, our current research activities have also been focused on designing strategies to modulate tumor microenvironment with the potential for synergizing the outcome of conventional therapeutic approaches and developing a better understanding of the role of tumor-stromal interaction in tumor progression and metastasis.
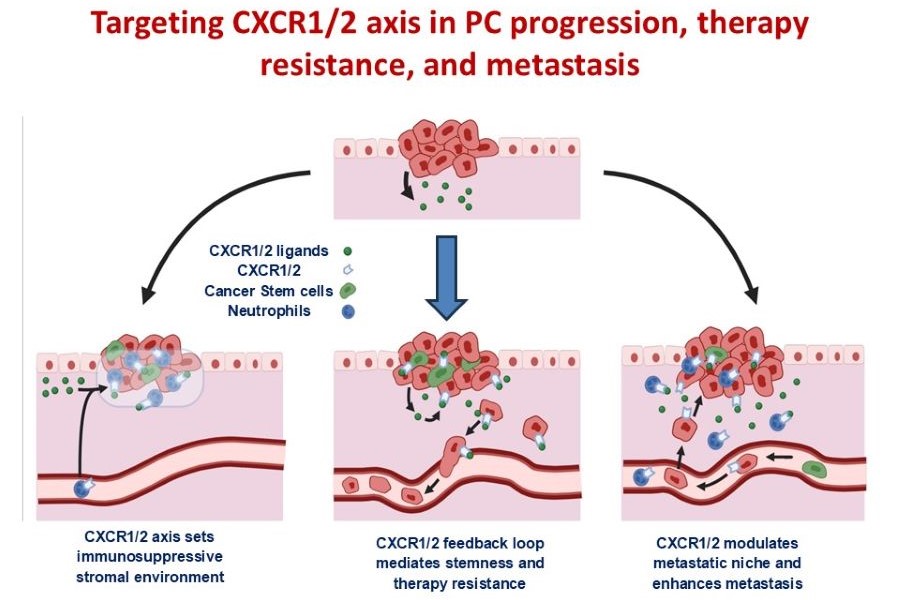
Targeting CXCR1/2 axis in PC progression, therapy resistance, and metastasis
Molecules driving a tumor-associated proinflammatory and pro-metastatic phenotype in pancreatic cancer (PC) have considerable potential as therapeutic targets, yet this area remains relatively under-explored. Our central hypothesis is that targeting the CXCR1/2 signaling will counter the tumor microenvironment-mediated mechanisms of therapy resistance and reduce metastatic dissemination of pancreatic cancer cells. With pharmacologic inhibitors of CXCR1/2 already in clinical trials, the rapid development of innovative therapies targeting CXCR1/2 in combination with conventional anti-tumor regimens is a genuine possibility and can be added to ongoing clinical trials for PC.
PC-Neutrophil interaction in therapy resistance and metastasis
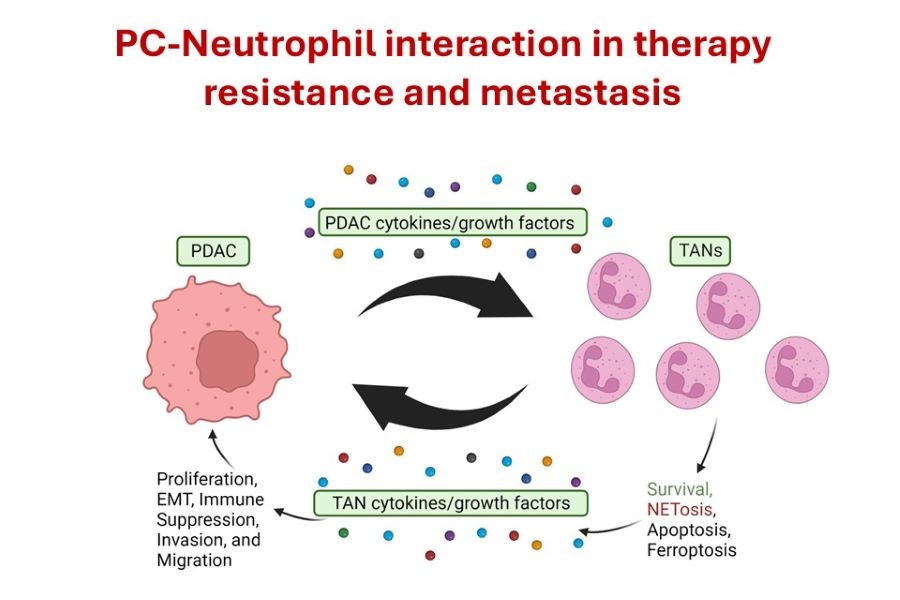
Neutrophils are a pro-tumorigenic and pro-metastatic part of the TME, aiding PDAC in progression and therapy resistance; however, the mechanisms by which neutrophils are recruited and activated remain unclear. The specific objective is to determine the role of
tumor-associated neutrophils in PDAC progression, therapy resistance, and metastasis. The working hypothesis is that neutrophils play a critical pro-tumorigenic and pro-metastatic role in the progression and therapy resistance in PDAC. We are focused on: 1) Defining the functional significance of neutrophil recruitment, activation, and survival during PDAC progression and therapy resistance; and 2) Evaluating whether targeting tumor-associated neutrophil recruitment/activation inhibits progression and enhances therapeutic responses.
Osteotropic targeting of Cathepsin G in bone metastasis
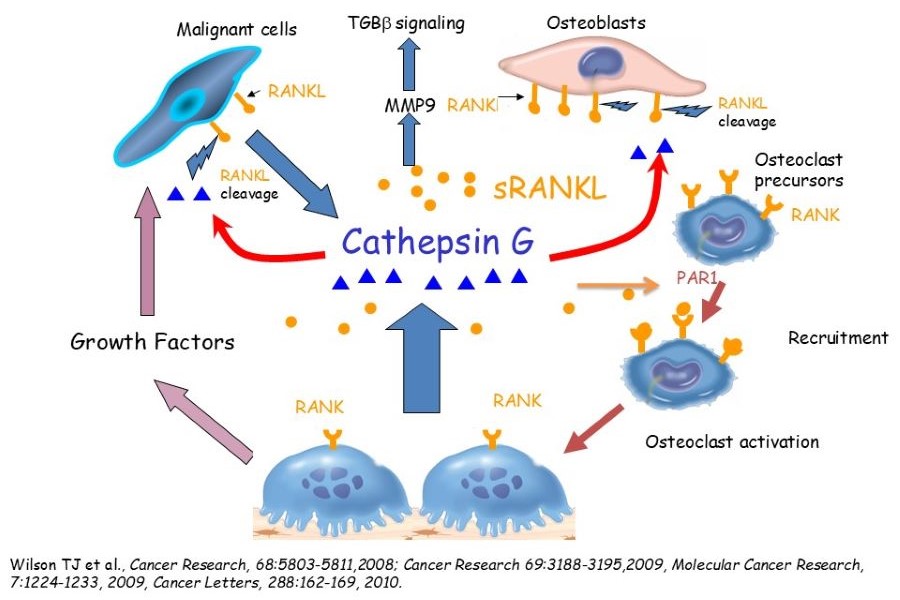
Understanding the cellular and molecular changes in the bone microenvironment is essential for developing novel therapeutics. We have identified Cathepsin G (CTSG) up-regulation as a critical regulator of tumor-bone interaction and CTSG's functional role in osteolytic bone metastasis through the generation of soluble RANKL.
Additionally, we have developed osteolysis targeting nanomedicine to deliver CTSG inhibitors and docetaxel. We are testing whether targeting CTSG expression and/or activity associated with docetaxel in the osteolytic bone microenvironment will inhibit bone metastasis and improve survival. Our hypothesis is that inhibition of sRANKL generation at the tumor bone (TB)-interface combined with chemotherapy in the osteolytic bone microenvironment will abrogate the establishment of breast cancer bone metastasis.
Our rationale for these studies is that developing novel approaches to modulate molecular targets' expression critical in tumor-bone interaction and targeted delivery of a chemotherapeutic drug to the osteolytic microenvironment with limited toxicity would establish a robust scientific framework for eventual clinical trials in humans.
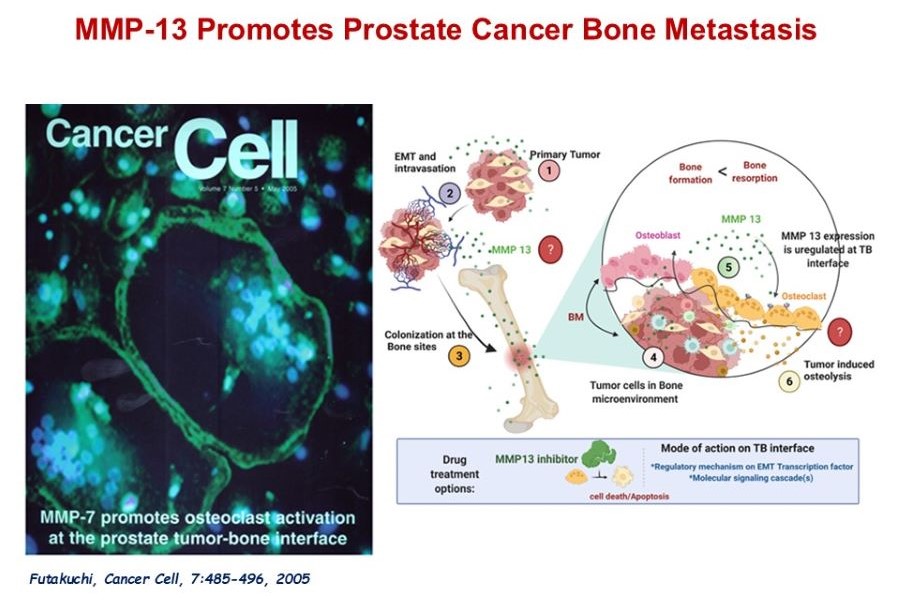
MMP13 in Prostate Cancer Bone Metastasis
Our working hypothesis is that malignant cells exploit the normal homeostatic process of bone remodeling. Using our unique animal model and proteases-specific microarray analysis, we have identified several candidate genes, matrix metalloproteinase (MMP)-7, MMP9, and MMP13 were aberrantly expressed in the tumor bone microenvironment. Our earlier study has demonstrated the functional significance of one such identified gene, MMP-7, as critical for prostate cancer-induced osteolysis. Currently, we are working on defining the role of MMP13 in prostate cancer-induced-osteoblastic/osteolytic changes and the establishment of bone metastases. We anticipate that these studies will lead to the development of innovative approaches to inhibit osteoblastic/osteolytic prostate cancer bone metastasis and malignant cell proliferation in the bone microenvironment using our novel delivery system, to develop a highly effective therapy for prostate cancer bone metastasis with minimal toxicity.
Lab Personnel

Ridhi Bhola, MDS, Graduate Student
Raina Barnes, M.S., Graduate Student
Esther Johnason, M.S., Graduate Student
Rachel Soukup, B.S., Graduate Student
Reagan Sturgeon, B.S., Graduate Student