Michele Plewes, PhD

Research Interests
- Gonadotrophin signaling
- Ovarian aging
- Lipid metabolism (sterol metabolism and lipid droplet biology)
- Mitochondrial function
Understanding Mitochondria, Lipid Signaling, and Reproductive Health
Mitochondria play a central role in cell biology and medicine, responding dynamically to genetic, metabolic, and hormonal signals. My research focuses on the intersection of mitochondrial function, lipid signaling, and reproductive health, with the goal of uncovering how hormones regulate sex steroid synthesis and influence fertility. By exploring the molecular mechanisms behind progesterone and testosterone production, I aim to advance our understanding of fertility and ovarian health.
Driving Innovation in Reproductive Health
Through cutting-edge approaches like multi-omics, molecular biology, and cellular imaging, my research bridges basic science and clinical applications. By uncovering how mitochondria and lipids influence fertility, my lab contributes to advancing treatments for infertility and age-related reproductive challenges.
Current Research Projects
1. Gonadotrophic Signaling in Fertility and Steroid Synthesis
Infertility affects one in six couples worldwide, often linked to disruptions in sex steroid synthesis. My lab investigates how gonadotropic signals are transmitted to lipid droplets and mitochondria, driving critical processes like lipid metabolism and steroidogenesis. By deciphering these pathways, we aim to improve reproductive health outcomes and offer innovative therapeutic insights.
2. Lipid Signaling and Mitochondrial Dynamics During Ovulation
Ovulation is a pivotal phase of fertility, governed by endocrine signals that influence oocyte maturation and corpus luteum formation. We focus on how gonadotropin signaling, specifically through LHCGR, regulates lipid metabolism and mitochondrial biogenesis during ovulation and luteinization. This research provides new perspectives on anovulation and infertility in both humans and animals.
3. Mitochondrial Reprogramming in the Aging Ovary
With increasing maternal age, ovarian function declines, leading to reduced fertility and oocyte quality. My lab explores how granulosa cells, vital for oocyte maturation and steroid hormone production, are impacted by age-related changes in sterol homeostasis and mitochondrial function. By understanding these mechanisms, we aim to develop strategies to combat age-related infertility and improve ovarian health.
Information
Graduate Training
PhD in Biological Education, University of Northern Colorado, Colorado
Post-Doctoral Fellowship
Reproductive Biology and Biochemistry
The Signal Transduction Laboratory, Department of Obstetrics and Gynecology
University of Nebraska Medical Center, College of Medicine, Omaha, Nebraska
See publications for Michele Plewes, PhD
(Disclaimer: This search is powered by PubMed, a service of the U.S. National Library of Medicine. PubMed is a third-party website.)
- 09/2023-08/2027
Metabolic and Mitochondrial Signals During Ovulation
USDA NIFA
2023-67015-40795 (PI: Davis; Co-I: Plewes, Przygrodzka, Murry, Cupp) - 07/2024-07/2025
Sterol Metabolism and Mitochondria in the Aging Ovary
Nebraska Center for Women’s Health Research (PI: Plewes; Co-I: Zeljka Korade) - 01/2024-01/2027
Olsen Center for Women’s Health – (PI: Plewes) - 07/2024-07/2025
SREBF1/2 Signaling in Granulosa Cells: Steroidogenesis, Lipid Metabolism, and Mitochondrial Function
Nebraska Research Collaboration Initiative (PI: Plewes; Co-I: Drs. Davis, Korade, Schott, and Khalimonchuk) - 05/2023-05/2024
ShEEP Request for a Confocal Laser Scanning Microscope
US Department of Veterans Affairs
821-PS-45220 (PI: Bennett; Co-I: Plewes, McVicker, Viswanathan, Casey, Kharbanda) - 01/2021-01/2026
Mitochondria Dynamics and Steroidogenesis
VA-Career Development Award 2
1 IK2 BX004911-01 (PI: Plewes)
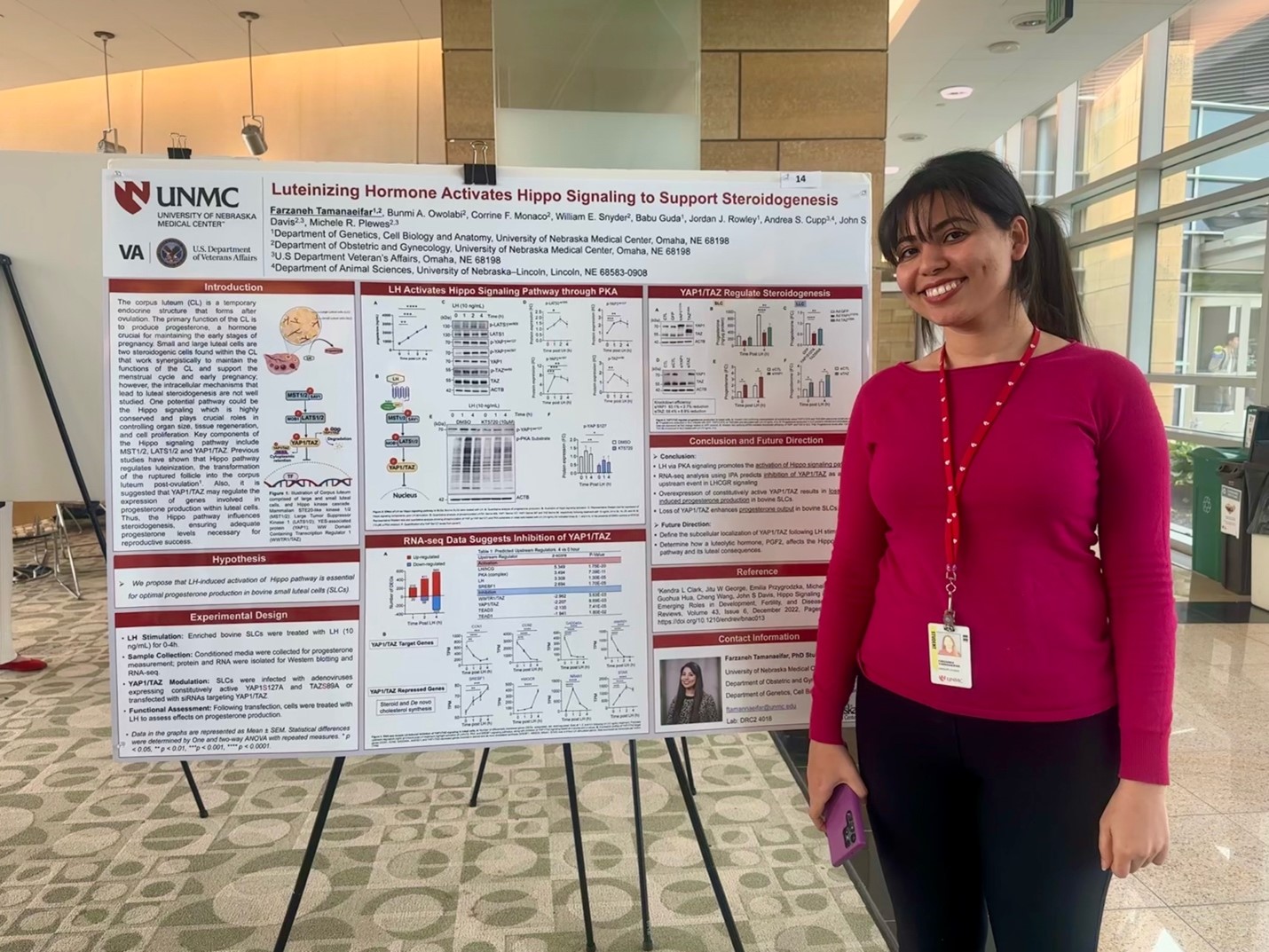
Farzaneh Tamanaeifar, PhD Student - VA 100 Years of Research Celebration
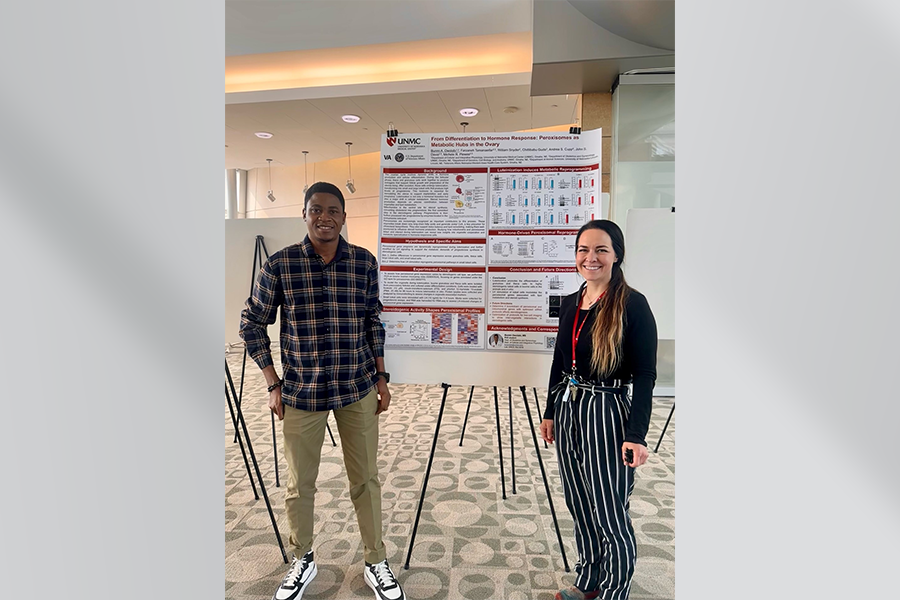
Bunmi Owolabi, PhD student, and Michele Plewes, PhD - VA 100 Years of Research Celebration

Farzan Tamanaeifar, PhD student (VA Research Week 2024)

Gracyn Little (SURP student 2024)

Plewes, Davis, and Clark Lab
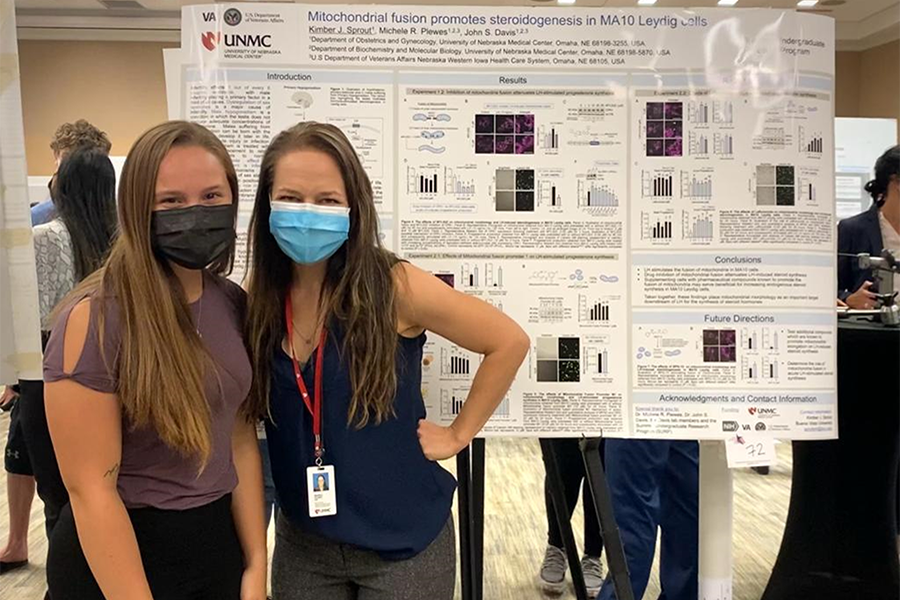
Kimber Sprout (SURP student 2022)
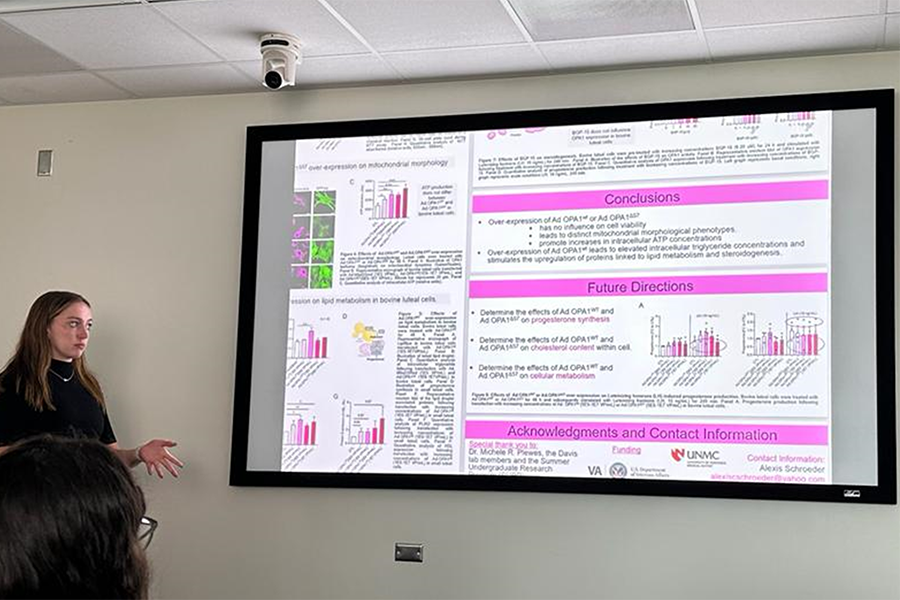
Alexis Schrouder presenting her summer research for the Dept. of OB-GYN (SURP student 2023)
Alexis Schroeder (SURP student 2023)