Home
—
College of Nursing
—
Research & Scholarship
—
Research & Innovation in Action
—
BEST-ICU Clinical Trial
—
For Providers
For Providers
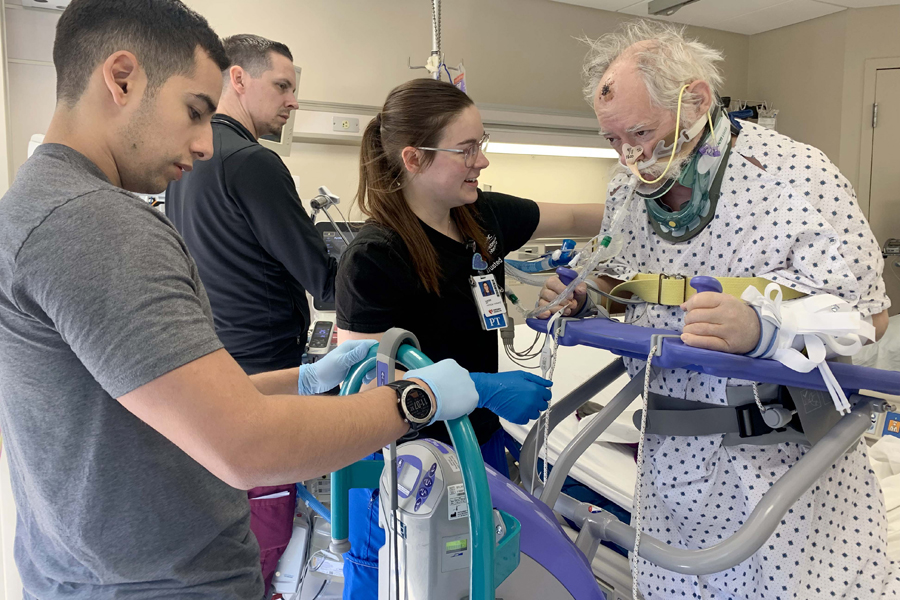
Welcome to the resource page for intensive care unit (ICU) providers participating in the national research study called BEST-ICU. Your ICU is participating in this clinical trial along with many other ICUs throughout the country.
Why we are doing the study:
- Patients who require care in the ICU often have long-lasting health concerns. There is a set of evidence-based practices used in the ICU that can help patients recover, known as the ABCDEF bundle.
- While we know the ABCDEF bundle helps improve patient outcomes, it is often difficult to deliver in everyday ICU practice.
- This study's purpose is to evaluate two different ways of helping ICU providers increase ABCDEF bundle delivery to critically ill patients.
Things we might do differently during this study:
- This study will use one of two ways to increase ABCDEF bundle delivery, either:
Unit Dashboard - An in-unit video screen to remind hospital teams to do required care tasks for a patient.
Nurse Facilitator - A dedicated nurse who will work with the ICU team to help with tasks like getting patients moving, monitoring their progress, and setting goals.
- Randomization of these strategies depends on study timing and in which ICU you are a provider.
- All patients will continue to get care as usual as ordered by the ICU providers.
What does this mean for providers in the ICU:
- Researchers are collecting information about care processes and patients in the ICU, but they will make sure that the information is kept private and safe.
- To protect your confidentiality, the information sent to the BEST-ICU research team will be identified by a scrambled code. No patients will be able to be identified by name in the study records.
- As a provider, you may also be asked about the acceptability of these strategies. If so, you will be given more information about that during an informed consent process.
Contact Us


IRB# 0794-23-FB