Situation: Patients with pacemakers and/or ICDs are frequently scheduled for procedures, including MRIs.
Background: There is a Nebraska Medicine policy, NM MS49 Surgical Patients with Cardiac Implantable Electronic Devices (CIEDs), and an order available for pacemaker and/or ICD interrogation.
Assessment: All patients with a CIED are to be identified preoperatively and the device clinic and staff are to be notified. Devices will be interrogated and reprogrammed as clinically indicated when the patient is in the pre-op area. Placing the order allows for additional communication with the device team.
- Access the order set by entering a keyword. “Pacemaker” or “ICD” will work.
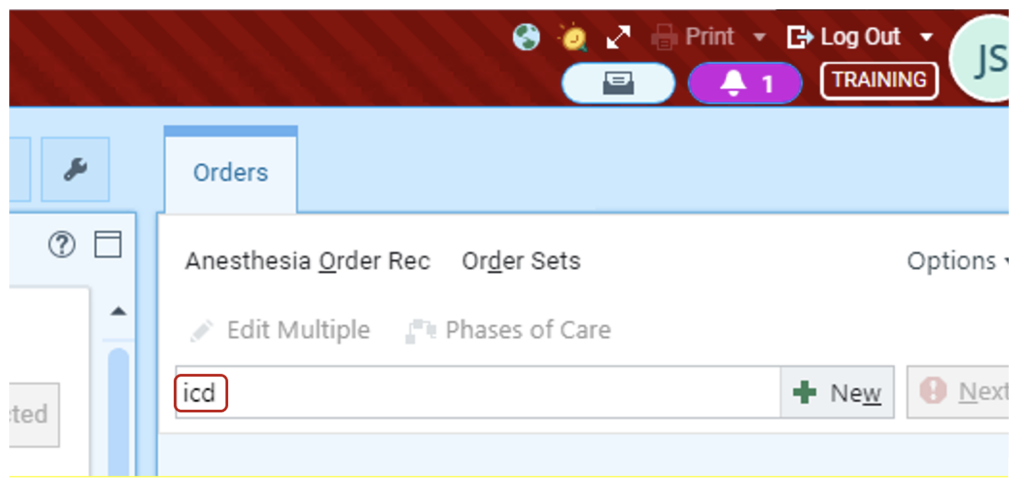
2. Choose “Pacemaker/ICD interrogation”
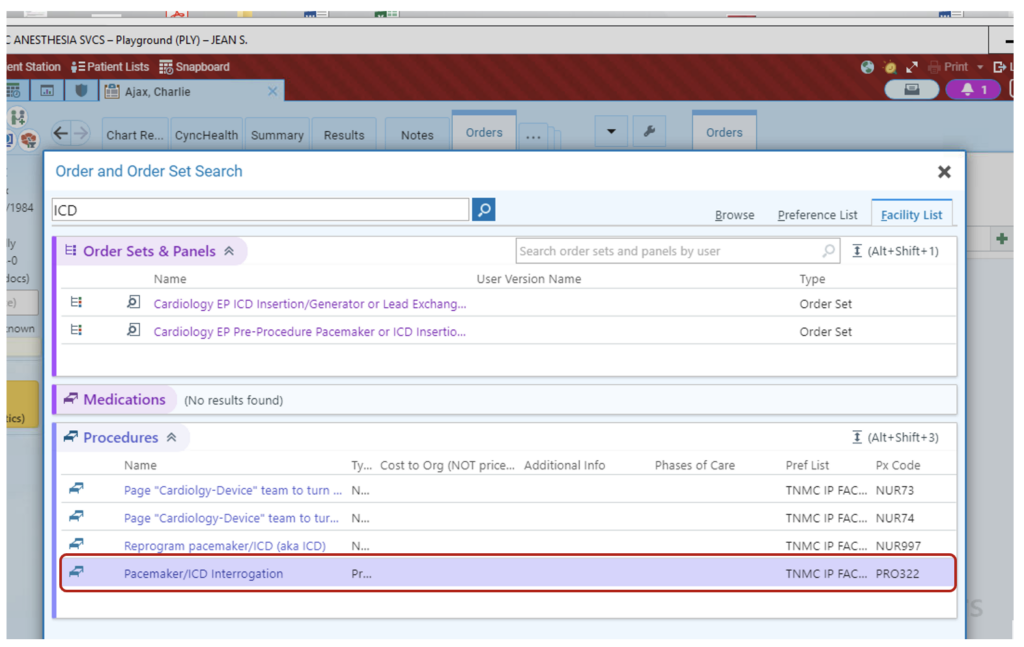
3. Select “Peri Procedural Evaluation/Reprogramming” for evaluation prior to the procedural case, including MRI. Then select routine or stat, and add any comments, such as type of case, that will help guide evaluation.
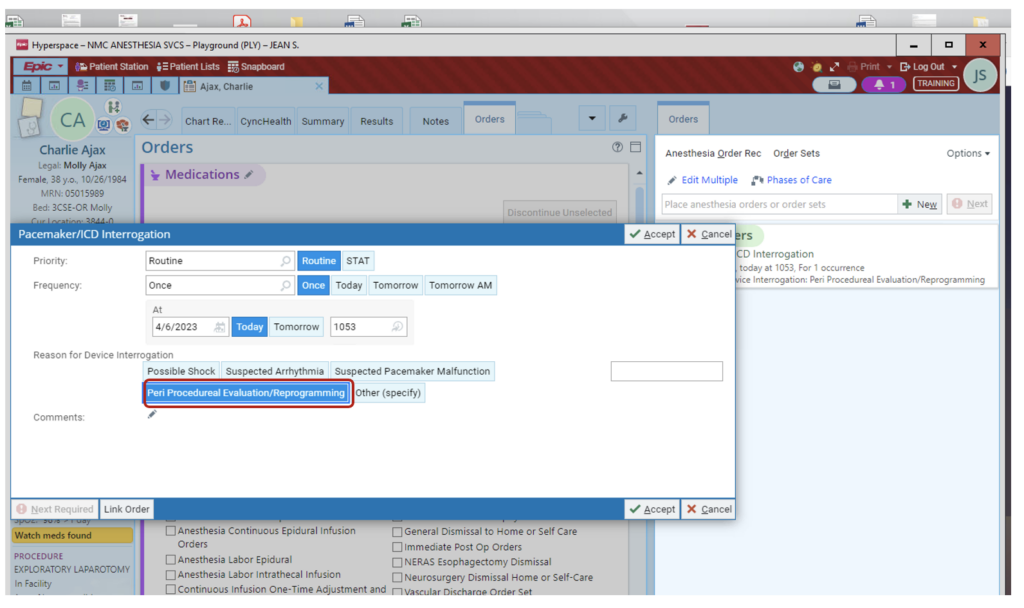
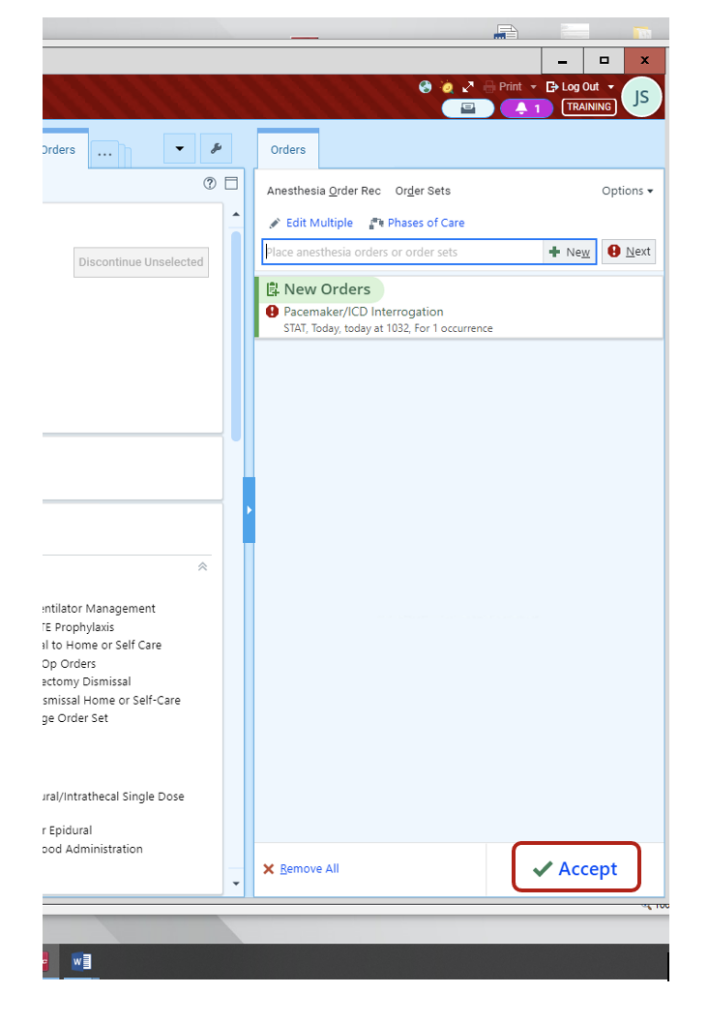
4. Once all fields are modified/selected/reviewed, Accept order.
Recommendations:
- Device interrogation orders can be placed with other preop orders; this may occur when a patient is seen in PECC. Preop staff will release.
- Verify with the preop team that the device team has been notified. The device team may request to discuss the case with the anesthesia team, including the location of the device, procedural details, utilization of cautery, etc.
- Review interrogation notes and recommendations; clarify any questions or concerns with the device nurse or cardiology electrophysiology attending.
- A prior device interrogation may or may not be appropriate for a peri-procedural interrogation. The device interrogation team will clarify.
- If the patient has an ICD and tachy therapies are disabled, the patient MUST have an external defibrillator applied by the nursing staff before deactivation of any tachy therapies of the ICD.
- If device settings have been modified for the case, ensure the device is re-set in PACU after the conclusion of the case.
- Refer to NM policy MS49 for additional details.