Jim Shull, Ph.D., a professor at UNMC’s Eppley Institute for Research in Cancer and Allied Diseases, was recently introduced at the University of California-Berkeley as the man who made the ACI rat famous.
 In a series of studies published over the past five years, Dr. Shull and colleagues have demonstrated that the ACI rat provides a valuable experimental model for studying the mechanisms through which estrogens induce breast cancer development.
In a series of studies published over the past five years, Dr. Shull and colleagues have demonstrated that the ACI rat provides a valuable experimental model for studying the mechanisms through which estrogens induce breast cancer development.
According to Dr. Shull, it has been widely known for many years that estrogens are a critical factor in the development of breast cancer.
This knowledge arose from studies that indicated a decrease in breast cancer risk in women who, for a variety of medical reasons, had their estrogen-producing ovaries removed before menopause.
More recently, it was demonstrated that the risk of breast cancer is significantly decreased in women who have used Tamoxifen, a drug that blocks the actions of estrogen and, in turn, inhibits the growth of breast cancer cells.
 Although these indications of the role of estrogens in breast cancer are important, further studies need to be performed to reveal the mechanisms through which estrogens contribute to breast cancer. Understanding these mechanisms should reveal more effective strategies for treating and preventing breast cancer.
Although these indications of the role of estrogens in breast cancer are important, further studies need to be performed to reveal the mechanisms through which estrogens contribute to breast cancer. Understanding these mechanisms should reveal more effective strategies for treating and preventing breast cancer.
For these reasons, it became imperative to find an animal model in which development of breast cancer is triggered by the same factors believed to be important in humans, most importantly estrogens. That is what Dr. Shull and his colleagues did.
These investigators discovered that breast cancers develop within approximately six months in 100 percent of female ACI rats treated with a specific estrogen called estradiol.
In contrast, it has been demonstrated that two other strains of laboratory rats, Copenhagen and Brown Norway, are resistant to estradiol-induced breast cancer.
Recognizing this unique susceptibility of the ACI rat to estradiol-induced breast cancer, Dr. Shull’s laboratory set out to discover why this animal was so different from the others.
The first step in this process was to perform genetic studies in which the ACI rat was mated to either the resistant Copenhagen or Brown Norway rat, to determine if the susceptibility of the ACI rat to estradiol-induced mammary cancer would be inherited by its offspring.
Female progeny resulting from these genetic crosses did develop breast cancer nearly 100 percent of the time when treated with estradiol, indicating that genes play an important role in determining whether or not estrogens can induce breast cancer in this rat model.
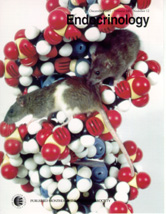
The first report on this work was published in the scientific journal Endocrinology in December of 2001, and a photograph of an ACI and Copenhagen rat sitting on a model of DNA double helix was featured on the cover of that issue.
Dr. Shull and colleagues have subsequently determined the locations within the rat chromosomes of at least four genes that account for the differing susceptibilities of the ACI, Copenhagen and Brown Norway rats to estrogen-induced breast cancer.
These findings are important, says Dr. Shull, because they lay the groundwork for ultimately identifying the genes that are responsible for breast cancer susceptibility in humans.