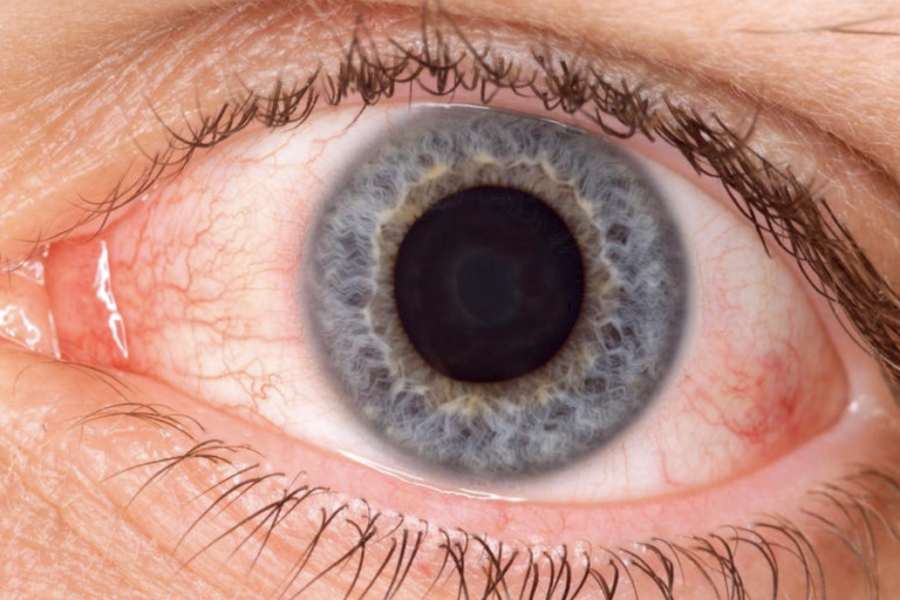
New Covid strain XBB.1.18, nicknamed arcturus, is quickly spreading across the U.S., but experts are warning that pink eye and high fever, two symptoms of the new variant, are particularly present among children.
Arcturus is a subvariant of the highly contagious omicron variant, which is the most prevalent variant in the U.S., according to the Centers for Disease Control and Prevention.
The Centers for Disease Control and Prevention has seen an uptick in this variant among travelers—the week of March 6, it made up 1.1% of cases among travelers, and by the week of April 3, it made up 19.8%.
As of the week of April 22, the CDC reports arcturus makes up 9.6% of all U.S. cases, the second most prevalent subvariant behind XBB.1.5., which makes up 73.6% of cases.
The World Health Organization is tracking arcturus and labeled it a “variant of interest” alongside XBB.1.5. on April 17, a step below a variant of concern—WHO previously labeled it a “variant under monitoring” in January.
WHO also reports the subvariant has been detected in 33 countries, including the U.S., Australia, Singapore, Libya, Iran, Kuwait and Qatar, but it’s the most prevalent in India.
How do you treat this covid variant?
Hi Gloria, thanks for commenting. Same as we treat others – nirmatrelvir/ritonavir (Paxlovid) orally is the preferred treatment. Remdesivir intravenous (3 daily infusions) is a good back-up.
James Lawler, MD, MPH, FIDSA
Global Center for Health Security
University of Nebraska Medical Center
Thank. You.
As with all infectious diseases, the tests we use for diagnosis of COVID-19 are not perfect, and some are more lesser-than-perfect than others. Antigen tests have dramatically lower sensitivity than PCR, and we see false negative tests quite frequently with antigen tests. Even with PCR, my colleagues and I have seen many cases with one or more negative PCR tests before we get a positive – in cases who had COVID-19 without a doubt. This is why clinical judgement, understanding the clinical features of the patient, and knowing the nuances of test performance all play a role in accurate diagnosis.
James Lawler, MD, MPH, FIDSA
Global Center for Health Security
University of Nebraska Medical Center
It happens. Sometimes, one test is not enough. And, as always, the antigen tests are far inferior in terms of sensitivity.
James Lawler, MD, MPH, FIDSA
Global Center for Health Security
University of Nebraska Medical Center
Test again day 3-5 as this variant is showing negative tests up until at least the 3rd day of active symptoms (fever, body aches). One patient with conjunctivitis symptoms had tested negative up until 10 days later
I spoke with Pfizer today about that exact issue. You may be interested to know that Paxlovid has not been reviewed yet for the current variant. So what we now have is a medication being prescribed under emergency use authorization EUA in a world in which the emergency has been declared over. There is no clear definition anymore of what constitutes FDA approval on Paxlovid.
Hi John, thank for your comment. Paxlovid is FDA approved – it is no longer being prescribed under emergency use authorization for adults.
https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-antiviral-treatment-covid-19-adults#:~:text=Today%2C%20the%20U.S.%20Food%20and,19%2C%20including%20hospitalization%20or%20death.
While it is true that SARS-CoV-2 variants now are genetically drifted considerably from original strains, the evolutionary changes are driven much more by immune response, antibody binding, and cell receptor binding. Therefore, one expects much more action at the spike protein (particularly receptor binding domain) than in other genes such as protease (the target for the drugs in Paxlovid). This appears to be true in genomic analysis. It is also true that computer modeling can predict which mutations can cause changes in protease significant enough to impact drug activity.
Finally, we have the analogy of flu, where we don’t have to redo clinical trials of antivirals like oseltamivir (Tamiflu) or other neuramindase inhibitors every time we get a new dominant strain of influenza virus.
However, the point is still valid and well-taken. We only have solid clinical trial data for older variants, and we need to be vigilant for signs of concerning mutations in protease gene and/or indications that clinical efficacy may be diminished. Thankfully, so far, we haven’t seen that.
James Lawler, MD, MPH, FIDSA
Global Center for Health Security
University of Nebraska Medical Center
Just flew. Two days later pink eye started. One week later tested positive. Throat is bad now. So weird!
My daughter had extreme pinkeye and temporary blindness in her right eye and hives all over her body 1 week after positive covid test, she’s 23. Only feeling better now, it’s been a week. This is one heck of a virus.