The causes of long COVID, which disables millions, may come together in the brain and nervous system.
Tara Ghormley has always been an overachiever. She finished at the top of her class in high school, graduated summa cum laude from college and earned top honors in veterinary school. She went on to complete a rigorous training program and build a successful career as a veterinary internal medicine specialist. But in March 2020 she got infected with the SARS-CoV-2 virus—just the 24th case in the small, coastal central California town she lived in at the time, near the site of an early outbreak in the COVID pandemic. “I could have done without being first at this,” she says.
Almost three years after apparently clearing the virus from her body, Ghormley is still suffering. She gets exhausted quickly, her heartbeat suddenly races, and she goes through periods where she can’t concentrate or think clearly. Ghormley and her husband, who have relocated to a Los Angeles suburb, once spent their free time visiting their “happiest place on Earth”—Disneyland—but her health prevented that for more than a year. She still spends most of her days off resting in the dark or going to her many doctors’ appointments. Her early infection and ongoing symptoms make her one of the first people in the country with “long COVID,” a condition where symptoms persist for at least three months after the infection and can last for years. The syndrome is known by medical professionals as postacute sequelae of COVID-19, or PASC.
People with long COVID have symptoms such as pain, extreme fatigue and “brain fog,” or difficulty concentrating or remembering things. As of February 2022, the syndrome was estimated to affect about 16 million adults in the U.S. and had forced between two million and four million Americans out of the workforce, many of whom have yet to return. Long COVID often arises in otherwise healthy young people, and it can follow even a mild initial infection. The risk appears at least slightly higher in people who were hospitalized for COVID and in older adults (who end up in the hospital more often). Women and those at socioeconomic disadvantage also face higher risk, as do people who smoke, are obese, or have any of an array of health conditions, particularly autoimmune disease. Vaccination appears to reduce the danger but does not entirely prevent long COVID.
The most common, persistent and disabling symptoms of long COVID are neurological. Some are easily recognized as brain- or nerve-related: many people experience cognitive dysfunction in the form of difficulty with memory, attention, sleep and mood. Others may seem rooted more in the body than the brain, such as pain and postexertional malaise (PEM), a kind of “energy crash” that people experience after even mild exercise. But those, too, result from nerve dysfunction, often in the autonomic nervous system, which directs our bodies to breathe and digest food and generally runs our organs on autopilot. This so-called dysautonomia can lead to dizziness, a racing heart, high or low blood pressure, and gut disturbances, sometimes leaving people unable to work or even function independently.
The SARS-CoV-2 virus is new, but postviral syndromes are not. Research on other viruses, and on neurological damage from the human immunodeficiency virus (HIV) in particular, is guiding work on long COVID. And the recognition that the syndrome may cause its many effects through the brain and the nervous system is beginning to shape approaches to medical treatment. “I now think of COVID as a neurological disease as much as I think of it as a pulmonary disease, and that’s definitely true in long COVID,” says William Pittman, a physician at UCLA Health in Los Angeles, who treats Ghormley and many similar patients.
Although 16 million U.S. sufferers is a reasonable estimate of the condition’s toll, there are other, more dire assessments. A meta-analysis of 41 studies conducted in 2021 concluded that worldwide, 43 percent of people infected with SARS-CoV-2 may develop long COVID, with about 30 percent—translating to approximately 30 million people—affected in the U.S. Some studies have offered more conservative numbers. A June 2022 survey reported by the U.S. National Center for Health Statistics found that among adults who had had COVID, one in five was experiencing long COVID three months later; the U.K. Office for National Statistics put the estimate at one in 10. Even if only a small share of infections result in long COVID, experts say, they will add up to millions more people affected—and potentially disabled.
Most of the first recognized cases of long COVID were in patients who needed extended respiratory therapy or who had obvious organ damage that caused lasting symptoms. People reporting neurological symptoms were often overlooked or dismissed as traumatized by their initial illness and hospitalization. But as 2020 came to an end, says Helen Lavretsky, a psychiatrist at the University of California, Los Angeles, “we started getting to a place of sorting through what was really going on … and it became very evident at that time that neuropsychiatric symptoms were quite prevalent,” most commonly fatigue, malaise, brain fog, smell loss and post-traumatic stress disorder, as well as cognitive problems and even psychosis.
Ghormley was in her late 30s and relatively healthy when she caught the virus, but she had underlying conditions—including rheumatoid arthritis and asthma—that put her at risk for severe COVID. She spent several days at home, struggling to breathe, and then she went to the hospital, where her blood pressure soared and her blood glucose dropped precipitously. She mostly recovered from this acute phase within a few weeks, but, she says, “I never really got better.”
Soon after coming home from the hospital, Ghormley developed what her husband called “goldfish brain.” “I’d put something down and have no idea where I put it,” she recalls. “It kept happening over and over. I was thinking, ‘This is getting weird.’ My husband said I was not remembering anything. I’d try to talk, and I knew what I wanted to say, but I couldn’t think of the word.”
She also experienced tremors, dramatic mood swings and painful hypersensitivity to sounds. “My husband opening a paper bag felt like knives stabbing me in the ear,” she recounts. Any exertion—physical or mental—left her exhausted and in pain. The changes were jarring to Ghormley, who prided herself on her sharp mind. “The thing that bothered me the most was that I was really having trouble thinking, speaking, remembering—trying to complete a task and having no idea what it was. Suddenly I had quite profound neurological deficits. Everything fell apart for me at that time. That was horribly traumatic … it kind of broke me. I didn’t feel like me.”
ROOTS OF DYSFUNCTION
As a veterinary internist, Ghormley says, it’s her job to problem solve when mysterious symptoms arise, including her own. “I was actively trying to find reasons and find what I could do.” She theorized that some of her neurological symptoms might be the result of thrombotic events, blood clots that can cause ministrokes. Several early studies showed that COVID attacks endothelial cells, which line blood vessels. That can lead to clotting and oxygen deprivation in multiple organs, including the brain. Even subtle disruption of endothelial cells in the brain could contribute to cognitive dysfunction.
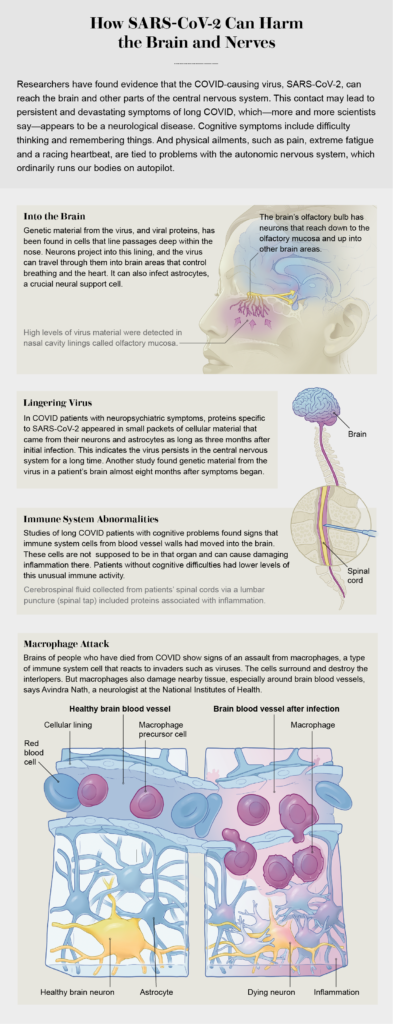
One study found that in people with neurological COVID symptoms, the immune system seems to be activated specifically in the central nervous system, creating inflammation. But brain inflammation is probably not caused by the virus infecting that organ directly. Avindra Nath, who has long studied postviral neurological syndromes at the National Institutes of Health, found something similar in an autopsy study of people who died of COVID. “When you look at the COVID brain, you don’t actually find [huge amounts of virus, but] we found a lot of immune activation,” he says, particularly around blood vessels. The examinations suggested that immune cells called macrophages had been stirred up. “Macrophages are not that precise in their attack,” Nath says. “They come and start chewing things up; they produce all kinds of free radicals, cytokines. It’s almost like blanket bombing—it ends up causing a lot of damage. And they’re very hard to shut down, so they persist for a long time. These are the unwelcome guests” that may be causing persistent inflammation in the brain.
Determining which patients have ongoing inflammation could help inform treatments. Early research identified markers that often are elevated in people with the condition, says Troy Torgerson, an immunologist at the Allen Institute in Seattle. Three cell-signaling molecules—tumor necrosis factor alpha, interleukin 6 and interferon beta—stood out in long COVID patients. But this pattern wasn’t found in absolutely everyone. “We’re trying to sort through long COVID patients and say, ‘This would be a good group to take to trials of an anti-inflammatory drug, whereas this group may need to focus more on rehabilitation,’” Torgerson says. He led a study (currently released as a preprint, without formal scientific review by a journal) in which his team measured proteins from the blood of 55 patients. The researchers found that a subset had persistent inflammation. Among those people, they saw a distinct immune pathway linked to a lasting response to infection. “One subset of patients does appear to have an ongoing response to some virus,” Torgerson says.
Isolated pockets of SARS-CoV-2 or even pieces of viral proteins may remain in the body well after the initial infection and continue to elicit an immune attack. The first solid evidence for “viral persistence” outside the lungs came in 2021 from researchers in Singapore who found viral proteins throughout the gut in five patients who had recovered from COVID as much as six months earlier. A study conducted at the University of California, San Francisco, found evidence for viral particles in the brains of people with long COVID. Scientists collected exosomes, or tiny packets of cellular material, released specifically from cells of the central nervous system. The exosomes contained pieces of viral proteins as well as mitochondrial proteins, which may indicate an immune attack on those vital cellular organelles. Amounts of such suspicious proteins were higher in patients with neuropsychiatric symptoms than in those without them.
The virus could linger in the brain for months, according to research conducted at the NIH and reported in Nature in December 2022. The autopsy study of 44 people who died of COVID found rampant inflammation mainly in the respiratory tract, but viral RNA was detected throughout the body, even in the brain, as long as 230 days after infection. Two other studies, both published last year in the Proceedings of the National Academy of Sciences USA, showed evidence that SARS-CoV-2 may infect astrocytes, a type of neural support cell, gaining entrance via neurons in the skin lining the nose.
Researchers are examining inflammatory signals in patients with long COVID in increasingly fine detail. A small study led by Joanna Hellmuth, a neurologist at U.C.S.F., found that patients with cognitive symptoms had immune-related abnormalities in their cerebrospinal fluid, whereas none of the patients without cognitive symptoms did. At the 2022 meeting of the Society for Neuroscience, Hellmuth reported that she had looked at more specific immune markers in people with cognitive symptoms and found that some patients had an elevated level of VEGF-C, a marker of endothelial dysfunction. Higher VEGF-C concentrations are associated with higher levels of immune cells getting into the brain, she says, and “they’re not doing their normal function of maintaining the blood-brain barrier; they’re distracted and perhaps activated.” Although the studies are small, Hellmuth adds, they reveal “real biological distinctions and inflammation in the brain. This is not a psychological or psychosomatic disorder; this is a neuroimmune disorder.”
What keeps the immune system in attack mode? According to Torgerson, “one option is that you’ve developed autoimmunity,” in which antibodies produced by the immune system to fight the virus also mark a person’s own cells for immune attack. The response to the virus “turns the autoimmunity on, and that doesn’t get better even when the virus goes away,” he says. Several studies have found evidence of autoimmune components called autoantibodies that interact with nerve cells in people with long COVID.
Clues about the inflammatory processes at work could point toward treatments for neurological symptoms. “If it’s a macrophage-mediated inflammatory process … intravenous immunoglobulin could make a difference [to] dampen the macrophages,” Nath says. The treatment, referred to as IVIg, contains a cocktail of proteins and antibodies that can mitigate an overactive immune response.
IVIg can also be used to block autoantibodies. And a therapy called rituximab that targets antibody-producing B cells provides “a time-tested therapy for a lot of autoantibody-mediated syndromes,” Nath says. Another strategy is to use corticosteroids to dampen immune activity altogether, although those drugs can be used for only a limited time. “That’s a sledgehammer approach, and you can see if it makes a difference. At least it gives you an idea that, yes, it’s an immune-mediated phenomenon, and now we need to find a better way to target it,” Nath says.
If the virus does hang around in some form, antiviral medications could potentially clear it, which might help resolve neurological symptoms. That’s the hope of scientists running a clinical trial of Paxlovid, Pfizer’s antiviral drug for acute COVID.
A CHRONIC FATIGUE CONNECTION?
Postviral syndromes have been documented for more than a century, arising after infection with viruses from HIV to the flu. Epstein-Barr virus, which causes mononucleosis, is one of several viruses linked to a condition called myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), which is estimated to affect at least one and a half million people in the U.S. ME/CFS bears striking resemblances to long COVID, with symptoms such as immune system dysregulation, fatigue and cognitive dysfunction. “One of the patterns we see is patients who definitely meet the criteria for ME/CFS. This is something we are seeing and treating all the time” in long COVID patients, Pittman says. ME/CFS can be severe, with some people losing mobility and becoming bedbound.
Nath, who also studies ME/CFS, says that “we think mechanistically they are going to be related.” Researchers suspect that ME/CFS, like some cases of long COVID, could be autoimmune in nature, with autoantibodies keeping the immune system activated. ME/CFS has been difficult to study because it often arises long after a mild infection, making it hard to identify a viral trigger. But with long COVID, Nath says, “the advantage is that we know exactly what started the process, and you can catch cases early in the [development of] ME/CFS-like symptoms.” In people who have had ME/CFS for years, “it’s done damage, and it’s hard to reverse that.” Nath speculates that for long COVID, if doctors could study people early in the illness, they would have a better chance of reversing the process.
Torgerson hopes that researchers will ultimately come to better understand ME/CFS because of COVID. “COVID has been more carefully studied with better technology in the time we’ve had it than any other infectious disease ever. I think we’ll learn things that will be applicable to other inflammatory diseases driven by infection followed by an autoimmune process.”
TEAM TREATMENT
Ghormley, after months of illness, sought care at UCLA Health’s long COVID clinic, among the country’s few comprehensive, multidisciplinary programs for people with this syndrome. Even though her symptoms are rooted in nervous system dysfunction, she needed an array of medical specialists to treat them. The clinic grew out of a program aimed at coordinating care for medically complex COVID patients, says its director Nisha Viswanathan, an internist and primary care physician. In following up with COVID patients after several months, she realized that “we had a group of patients who still had symptoms. There was no understanding around the condition; we were just trying to see what we could offer them.” Viswanathan and others convened a biweekly meeting of UCLA Health doctors in pulmonology, cardiology, neurology, psychiatry and other specialties to discuss individual cases and overall trends.
At UCLA Health, Pittman coordinates Ghormley’s treatment. He says the interdisciplinary team is crucial to getting patients the best possible care. “Oftentimes there are so many symptoms,” and some patients have seen multiple specialists before arriving, but not necessarily the right ones. As long COVID primary care providers, he says, “we do the initial testing and get them to the right person.” For Ghormley, that list of providers includes Pittman, along with a neurologist, a pulmonologist, a cardiologist, a psychiatrist, a trauma counselor, a rheumatologist and a gynecologist.
The team approach has also been critical for doctors trying to understand a brand-new disease, Pittman says. “It’s been a very interesting journey from knowing almost nothing to knowing a little bit now, and we’re learning more every day, every week, every month,” he says. The term “long COVID” “is an umbrella, and I think there are multiple diseases under that umbrella.” Although each long COVID patient is unique, Pittman says, “we start to see patterns developing. And with Ghormley, we saw a pattern of dysautonomia, which we see frequently.”
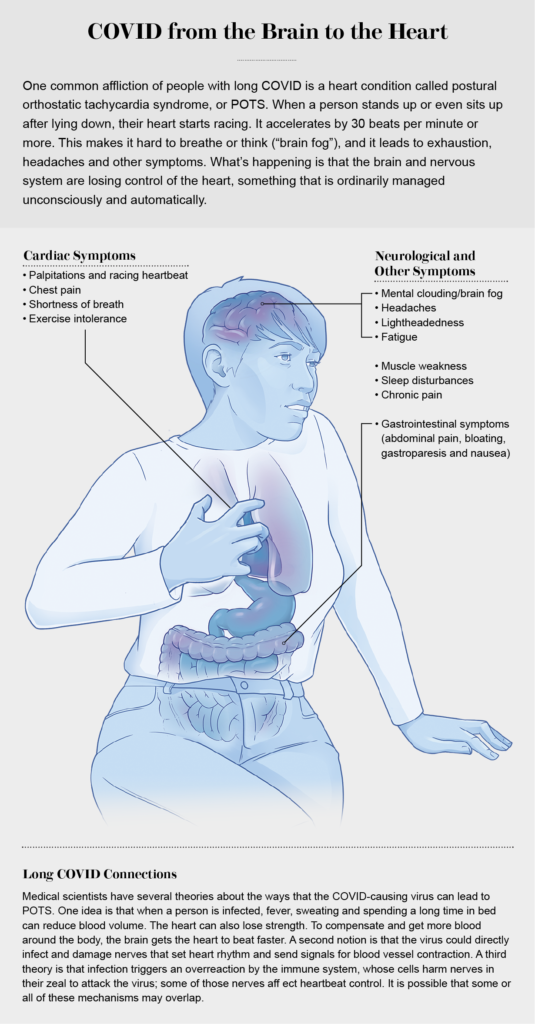
Dysautonomia impairs the autonomic nervous system, a network of nerves that branch out from the brain or spinal cord and extend through the body, controlling unconscious functions such as heartbeat, breathing, sweating and blood vessel dilation. For Ghormley, like many people with long COVID, dysautonomia takes the form of postural orthostatic tachycardia syndrome, or POTS. The syndrome encompasses a collection of symptoms that include a racing heart rate—particularly on standing—and fatigue, and it can cause bowel and bladder irregularities. POTS can also be a component of the exhaustion that comes with PEM. Although the symptoms may seem to affect the body, they stem from nervous system dysfunction.
Ghormley’s dysautonomia led her to see cardiologist Megha Agarwal at a UCLA clinic near her home. Many physicians are not familiar with POTS, but Agarwal is particularly attuned to it, having seen it in some of her patients before COVID hit. “There’s dysregulation of the nervous system, and so many things can cause it: some cancer therapies, viruses, autoimmune conditions.” Agarwal recognized POTS in Ghormley in the fall of 2020, when very little was known about long COVID. Now she believes “POTS is really what long-haul COVID is causing” in many patients. Luckily, Agarwal says, there are medical interventions that can help.
Tachycardia—the T in POTS—causes the heartbeat to speed up, contributing to exhaustion and fatigue in addition to stressing the heart itself. Drugs called beta-blockers (for the beta-adrenergic receptors they shut off in the heart) can lower the heart rate and improve symptoms. “When heart rate is controlled, not only does the pump improve,” Agarwal says, “[but people’s] energy improves, their fatigue is gone, and sometimes there’s better mental clarity.” For some patients like Ghormley, beta-blockers are not enough, so Agarwal adds a medication called ivabradine. “It’s a bit off-label, but it’s currently being aggressively studied” for POTS. For Ghormley, the combination led to real improvements, “so now she doesn’t feel like she ran the Boston Marathon when all she did was sit down and stand up at work or take a shower,” Agarwal says.
Among Ghormley’s toughest symptoms is her brain fog, a catchall term for a slew of cognitive problems that make it hard for her to function. For days when Ghormley works, her psychiatrist prescribes Adderall, a stimulant used to treat attention deficit hyperactivity disorder that helps her concentrate and stay focused. That has “helped immensely,” Ghormley says.
Ghormley credits her doctors and Agarwal in particular with doing the detective work to dig into her symptoms. “Nobody knew anything about it, but everyone listened to me,” Ghormley says. Perhaps because she was a professional from a medical field, no one “brushed me aside.”
That’s unusual for people with long COVID, many of them women, who are often dismissed by physicians who doubt their complaints are real. “Patients just don’t feel heard,” Viswanathan says. “I had a patient who told me everything, and after, I just said, ‘This must be so hard for you. I want you to know that everything you’re feeling is real, and I’ve seen so many patients like you.’ And she started crying. She said, ‘No one has told me that. I can’t tell you the number of times I was told it was in my head.’”
In addition to drugs, other types of therapies, including physical therapy, can help improve some symptoms. But people who experience PEM face a particular challenge when using movement therapies. Pittman says the exertion can make these patients feel worse. “We don’t want patients to go to not moving at all, but sometimes the type of movement they’re doing may be flaring their symptoms.” He notes that often PEM strikes young, previously healthy people who will say, “‘I need to push myself,’ and then they go way too far and get worse. Our job is to try to find that middle ground and then make that consistent over time, so they’re not getting further deconditioned but they don’t have the PEM, which has been shown to set them back.”
THE LONG HAUL
Some patients, Pittman says, “have the expectation that they’re going to come in, and within a month they’re going to be back to normal. And resetting those expectations can be really challenging. You have to be really empathetic because people’s lives have completely changed.” But sometimes patients’ quality of life can improve noticeably when they are able to adjust to a new normal. Still, he says, “patients have so many questions, and I can’t lead them down a physiological pathway. I can tell them there’s neuroinflammation, maybe there’s autoimmunity, but we still don’t have the answers. Sometimes it’s really tough for us to accept and for the patient to accept that we just have to try our best.”
A number of people, Viswanathan says, benefit from reducing various treatments they have accumulated. Some people become so desperate that they will try anything from supplements to off-label medications to untested potions from the Internet. Stopping those sometimes leads to improved symptoms, she says.
Psychological care and support groups can help. Lavretsky adds that “lifestyle choices can play a huge role in improvement,” particularly better sleep habits and the use of breathing exercises to control anxiety. She tells people their bodies can heal themselves if the patients and clinicians find the right tools.
Whether that’s true for everyone remains to be seen, Viswanathan says. “We see many patients who have gotten better with time. I have patients whose symptoms have disappeared in the course of a year, or they disappear and occasionally flare up again.” But for some, she says, “it could last many years.”
“We’re going to be addressing this for probably decades,” Viswanathan says. “COVID is not going to go away so much as we’re just going to get used to living with it, but part of [that] means that people will continue to develop long COVID.”
Vaccination appears to reduce the risk of long COVID. But a study published in May 2022 in Nature Medicine suggests the protection, though real, is not as good as one might hope. The survey of electronic health records from the U.S. Department of Veterans Affairs looked at the relatively small portion of vaccinated people who subsequently became infected. They developed long COVID only 15 percent less often than unvaccinated people. “These patients can have symptoms for one to two years or longer, and so every month you’re racking up more patients. Even if it’s 15 percent less, the total population of patients is still growing and exploding,” Pittman says. The best way to avoid getting long COVID, experts all agree, is to avoid getting COVID at all.
The syndrome is still mired in a lot of medical uncertainty. Patients might have one or a combination of the problems investigated so far: Long COVID might be caused by viral particles that persist in the brain or other parts of the nervous system. Or it might be an autoimmune disorder that lasts long after the virus has disappeared. Maybe overactive immune cells continue to perturb the nervous system and nearby blood vessels. Fortunately, the increasing ability to recognize specific problems is helping clinicians hone treatments that give patients the best chance of recovery.
Although Ghormley says her care has dramatically improved her symptoms and allowed her to “do some normal things again,” she continues to experience flare-ups that make it impossible for her to work for weeks at a time. One day last year she skipped a dose of her heart medication and made a Target run in the southern California heat. “I got home and basically collapsed in the hallway. Since then, everything has been out of whack. If I try to move around, my legs give out.” Most frustrating—and scary—to Ghormley is the unpredictability of her symptoms. “They have changed so much; some are manageable, some debilitating. One thing will get better, and another thing comes back. I’m always hopeful that it’s going to get better, but I just don’t know.”
This article was originally published with the title “The Brain and Long COVID” in Scientific American 328, 3, 26-33 (March 2023)
doi:10.1038/scientificamerican0323-26