Narrative description of Stem Cell Biology Research Laboratory activities
Present and Former Lab Members and Collaborators
|
|
|
|
|
|
|
|
Abby: Postdoc John's Hopkins Dr. Jackson: Assoc Prof., Wake Forest Institute Reg Med Sara: Medical Student, UNMC Dr. O'Kane: Assoc Prof. Dentistry, Creighton University Sonal: Houston, Texas Craig: Tech, Department of Surgery, UNMC Sue: Tech, Department Pharm Sci, UNMC Jordan: Medical Student, UNMC |
1. STEM CELLS AND AGING
a. Overview:
Aging is associated with declines in tissue repair and vitality, accompanied eventually, in many, but not all, individuals with cognitive decline. A proportion of the elderly population also develops frailty.
Stem cells are defined as cells that minimally maintain their own numbers, and at the same time give rise to at least one, and usually more than one, lineage of differentiating cells. This process gives rise to functional tissue cells (a differentiation hierarchy).
Stem cells reside in many tissues and are essential to tissue maintenance and repair and regeneration following injury in adults.
This project, involving a large investigative team (2004-2007), with Sue Brusnahan as lead technologist investigated whether declines in stem cell populations, either in numbers and/or quality underlies the process of aging. The behavior of normal stem cells is strictly regulated by microenvironmental stromal cell niches. This is necessary because of their potentially unlimited proliferative capacity. Stem cells are a risk to the individual (possible development of cancer) so organisms restrict the sites of stem cell proliferation. It is important to define if any decline in stem cells with aging is intrinsic to the stem cells or due to a decline in microenvironments or a combination of mechanisms.
Our future intent would be to intervene in a cost effective manner to maintain or enhance stem cell functions and thereby promote healthy aging.
Currently, physical activity/exercise is the best option to promote healthy aging, improve health in obese subjects and promote recovery from chemotherapy. Consequently, with Dr. Laura Bilek and Dr. Tim McGuire we are attempting to define the mechanisms by which physical activity/exercise has beneficial effects, so that the optimal regimens that benefit most individuals can be applied. (Current studies are funded by CAHP and a subproject of Dr. Carol Pullen’s (CON) NIH grant)
Specific Projects
b. Stem cell quality assays: Correlation with Aging/Health. (Supported by NIA # AG024912, 2004-2011)
This project, funded by the National Institute of Aging enrolled 233 human subjects since 2004. These individuals underwent hip replacement surgery. They were interviewed to define health and lifestyle factors. Their stem cell numbers and quality in blood and bone marrow were analyzed along with cytokine levels.
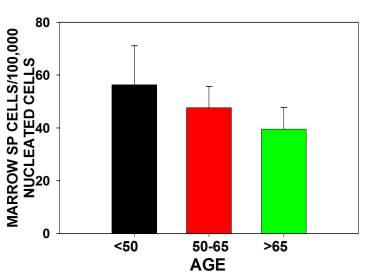
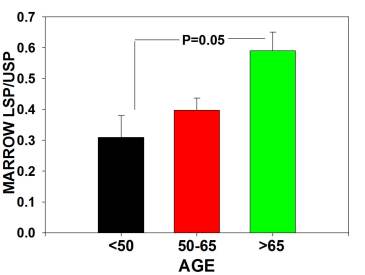
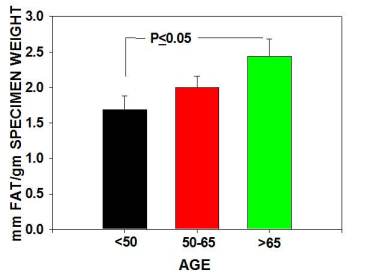
The major findings were that multipotential side population (SP) stem numbers declined with age (Figure 1A)but the quality of surviving stem cells was high (Figure 1B). Mesenchymal stem cell numbers declined in aging individuals. [Figures 2] As bone marrow fat increased with aging, stem cell numbers declined [Figure 3]. Obesity (as measured by increased Body Mass Index) was associated with evidence of inflammation and a decline in stem cell numbers.
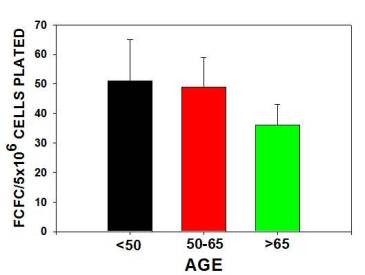
Sonal Tuljapurkar, showed that a lifestyle factor, alcohol consumption, induced the differentiation of mesenchymal stem cells to fat cells. This might compete with bone cells production and explain increased osteoporosis and delayed fracture repair in alcoholics. In addition, leptin, a hormone secreted by adipose tissue induced a rapid and more robust differentiation of MSC towards osteoblast lineage, potentially away from adipocytic lineage.
c. Physical activity, frailty, and stem cells: Two pilot studies were conducted, with Dr. Laura Bilek, assisted by technician Molly Lang, and Dr. Jane Potter respectively. A pilot study of physical activity and stem cells confirmed observations described above. Acute exercise was associated with a “mobilization” of stem cells into the circulation. In contrast, there was a trend to lower circulating endothelial cells but higher hemangiogenic cells numbers in individuals with higher levels of physical activity. We believe this might reflect responses to tissue stress in unfit individuals, versus compensation in physically fit individuals.
A pilot study of frail individuals compared to “healthy” elderly showed an amplified decline in stem cell populations. However, the age distribution of these populations was not comparable (the frail subjects were older).
d. Relationship between Physical Activity and Stem Cells: Dr. Bilek performed correlations between pedometer, accelerometer, HAP and BRFSS measures of physical activity to evaluate the potential relationship between various characteristics of h-PA and circulating numbers of stem cell populations. The mean circulating number of CD45-/CD31+ cells is higher in those individuals with h-PA levels above the median compared to those below the median. In contrast, mean circulating numbers of CD45-/CD31+/CD34+ and CD45+CD133+VEGFR2+ cells tended to be higher in persons categorized as low h-PA by most measures. These studies are being extended to analyze effects on immune cells.
e. Induced pluripotent stem cells (iPSC) as a tool to probe cellular and molecular mechanisms of aging. (This study was supported by State of Nebraska LB 606 funds, 2010-2012).
Since stem cell numbers decline with age, it is difficult to harvest significant numbers for analysis. This project was directed originally by Dr.Gayatri (Fnu) Sharma, then Dr. Partha Mukhopadhyay, assisted by Tracy Farrell, and generated IPS cells from donors of different ages, and employed different cell types as targets. This research showed that CD34+ cells from bone marrow proved to be the best reprogramming target. We are seeking funds to extend this analysis by using iPSC to repair damaged tissues.
2. CANCER STEM CELLS (Supported by NTSBRDF, 2010-2012)
a. Overview
Over the past 10 years, the postulate that cancers are maintained, metastasize and express therapy resistance based on rare cells with properties similar to tissue stem cells, has gained prominence. Unfortunately, many experiments approach this postulate as a self-fulfilling prophecy and it has been noted (Dr. P. Steeg, NIH) that there are more reviews than publications of relevant experimental data. If the postulate is correct, current therapies which primarily target the bulk of tumor cells may not be especially effective against cancer stem cells. Consequently, stem cells may preferentially survive current therapies and re-establish the tumor (relapse). This would potentially explain the difficulties in achieving treatment successes in many more prevalent cancers, as well as the high failure rate of new drugs in Phase I/II trials.
b. Do lymphomas have stem cells?
This project, funded by State of Nebraska Tobacco funds, applied techniques that identify normal tissue stem cells (Side population – SP, CD34+, CD133+, and aldehyde dehydrogenase hi cells) to cancers. Lymphoma cells represent an attractive cancer model because they express a phenotypic marker (CD20) and immunoglobulin gene rearrangements which serve as markers to track the cells in vivo in mice. Breast cancer is employed as a solid tumor model.
Cells with stem cell – like phenotypes and characteristics were isolated and analyzed in vitro for precursor cell frequencies and chemoresistance. The ability of these cells, compared to non-stem cells, to form tumors in immunodeficient mice was assessed.
Abby Heilscher, PhD (a former student; now a post doc at Johns Hopkins) showed Granta 519 cells, a mantle cell lymphoma line, has SP, CD34+, CD133+ and ALDH+ stem cells. The SP and CD133+ cells have a higher in vitro cobblestone area forming (stem) cell assay precursor frequency than non-SP or CD133 – cells, compatible with lymphoma stem cells. When transplanted into immunodeficient (NSG) mice, 5-10 SP cells compared to 104 or more non-SP cells formed tumors, compatible with SP “stem cells” being more tumorigenic.
When extracellular matrix Matrigel TM was added to SP or non-SP cells the precursor in frequency in vitro was much higher and equivalent. In vivo, as few as one SP and 10 non-SP cells formed tumors, suggesting microenvironmental factors were very important in tumorigenesis. Matrigel had no effect on tumor formation by CD133+ lymphoma cells.
These puzzling results are currently under further evaluation. Tumor formation requires vasculogenesis. SP versus non-SP and CD 133 cells were evaluated for production of an angiogenesis factor (IL-8). SP cells produce considerable amounts of IL-8, non-SP and CD133+ cells do not. This correlates with tumor formation. The role of factor production in tumorigenesis is under evaluation
c. Mechanisms of Lymphoma Chemoresistance: (This is a collaboration with Dr. Joshi).
The majority of cancer patients die because they have, or develop, therapy resistant disease. Earlier studies by a former MD/ PhD student, Colin Weekes (Section of Oncology, Department of Medicine, University of Colorado) showed that lymphoma contact with stromal cells promoted survival, inhibited apoptosis and induced chemoresistance. Additionally, chemoresistant lymphoma cell lines have been isolated by Dr. Joshi at UNMC. These lines have higher SP stem cell numbers and express higher levels of stem cell associated genes. Currently, this project is attempting to identify whether the genes associated with stromal associated therapy resistance are the same as intrinsically resistant cells and if the resistant cells have characteristics of stem cells. The intent is to determine if downregulation of these genes sensitizes the lymphoma cells to therapy and improves outcomes. Preliminary evidence suggests this is the case and Dr. Joshi is attempting to develop a Phase I/II clinical trial with oncologists Drs. Vose/Bierman and Genentech to develop this as a new and novel therapy.
d. Breast cancer stem cells and heterogeneity and interactions with stromal cells.
Initially, Gayatri (Fnu) Sharma, PhD and Partha Mukhopadhyay, PhD, Tracy Farrell, and Tim McGuire D. Pharm studied heterogeneity of breast cancer stem cells. These studies employed the murine Clone 66 orthotopic breast cancer cell line model that grows in syngeneic/immune intact mice. These cells have multiple cell types that express stem cell associated phenotypes, including all these described in the literature for breast cancer stem cells. These cells were flow sorted and compared for frequencies, growth, agar colony formation, mammosphere formation and size, response to growth factor containing Matrigel and limiting dilution in vivo tumor formation. The cells have also been evaluated for soluble factor production. The results, presented recently (8/12) at a Cold Spring Harbor Foundation Symposium show considerable heterogeneity. These data suggest that breast cancer cells with stem cell phenotypes, likely have a much more complex matrix organization than the current hypothesis of a simple linear branching stem cell hierarchy. (Please see an excellent review: Nguyen, et al., Nat. Reviews 12:133 (2012)).
3. MECHANISMS OF LUNG REPAIR (this is a collaboration with Dr. Rennard and his personnel, as well as Dr. Barb O’Kane and Tracy Farrell)
a. Overview
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death in the USA and is increasing in prevalence. Cigarette smoking is the primary cause of COPD worldwide. The defining feature of COPD is incompletely reversible airflow limitations during forced expiration, either from an increase in resistance of conducting airways or in lung compliance due to emphysematous destruction of alveolar walls. Several collaborative projects are in progress to investigate mechanisms of COPD and evaluate potential cellular therapies.
Specific Projects
a. Do cells from bone marrow, or the circulation, contribute to repair of emphysema?
These studies either transplanted bone marrow to a mouse receiving elastase intranasally to induce emphysema, or surgically joined the elastase recipient to a donor of marked (green fluorescent protein-expressing ) cells to see if these cells contributed to repair of emphysema. The donor cells did participate in repair and were integrated into the lungs of the recipients, but only in very small numbers (1% or less). Currently, we are trying, as yet unsuccessfully, to figure out how to increase this contribution. Retinoic acid treatment had minimal effects.
b. Autoimmune mechanisms in the development of emphysema.
In the previous experiments, a surprising observation was that in the surgically – joined mice (parabionts) the cell donor partner, not exposed to elastase, also developed emphysema. A search for mechanisms demonstrated that T cells from the mouse receiving elastase (i.e. a mouse developing emphysema) were capable of inducing emphysema in a naïve, untreated recipient. The mechanisms of this effect are under investigation as is the evaluation as to whether this mechanism is involved in cigarette smoking induced emphysema.
c. Mesenchymal Stem Cell (MSC) therapy of emphysema.
MSC ameliorate the tissue damaging effects of graft versus host disease in stem cell transplant recipients. MSC appear to be capable of suppressing inflammatory and immune reactions that damage tissues. We postulate that MSC administered to mice receiving elastase to create emphysematous damage may be protected by infusion of MSC. We are trying to identify a source of funding for this project.
4. ENGINEERING OF NANOBIOMATERIALS AND THEIR INTERACTIONS WITH STEM CELLS.
(Supported originally by NRI funds and more recently by DOE set-aside funds to Dr. F. Namavar, D.Sc., Orthopedic Surgery)
a. Overview:
Currently, there is a major interest in the application of stem cells in regenerative medicine. The objectives are to employ stem cells to generate new tissues or organs or repair damaged tissues. Generally, this requires three dimensionally interacting cellular structures. Attempts are underway to construct three dimensional scaffolds, e.g. electrospun scaffolds, to support bone formation by Dr. Russ Alberts, PhD (Omaha VA Hospital, currently at University of Southampton, UK) and to repair articular cartilage (A. Subramanian, PhD) at UNL. In addition there are searches for improved materials that interact productively, potentially instructively, with cells. This area of research, under the supervision of Dr. Fereydoon Namavar of the Department of Orthopedic Surgery with a MSIA graduate student, mechanical engineer Raheleh Miralami, is investigating triple smart nanosurfaces for coating of orthopedic prostheses. “Triple Smart” refers to surfaces that are hard, have outstanding wear characteristics, are compatible with cells for integration, and at the same time, inhibit bacterial growth.
Specific Projects
b. Nanostructured Metallic Oxide Coatings of Prostheses.
The main project is currently testing the hypothesis that nanostructured surfaces have differences in wettability and electrical charge. They can be structured to attract and bind fibronectin which, in turn, serves as a substrate for cell attachment, proliferation and differentiation. Such processes can promote the integration of a nanocoated prosthesis into tissue (e.g. bone), and improve fixation. Studies with a former student, Utsav Pokeval, showed that silver coatings were antibacterial and inhibited cell attachment and growth. Consequently in theory, by silver coating moving components of the prosthesis, they can be kept free of cells and bacteria and infections inhibited.
5. PHILOSOPHY AND MOTTO
The motto of the laboratory is “Stem cells in the service of mankind;” with an acknowledgement to Albert Einstein “If we knew what we were doing it wouldn’t be called research” Dr. Kessinger presented a plaque to us that has this quotation. We have a long track record of stem cell research. Our first bone marrow stem cell transplant in mice, at Birmingham University in England in mice in 1968, was performed the same year as the first successful bone marrow transplant in man. In a meeting in Cleveland 2002, Dr. Dirk Van Bekkum, one of this patient’s physicians reported that almost 40 years on, the patient is alive, has a family and is doing well.
Our objective is to extend this type of success to additional applications of stem cell therapies, to maintain the health of adult tissue stem cells and promote healthy aging and investigate how these processes go awry in cancer. Increasingly, the emphasis is to promote and assist in the development of younger investigators into successful independent faculty. Given funding constraints, this is likely the most challenging aspect of current activities.
6. RECENT PUBLICATIONS
For a list of recent publications, please see Dr. Sharp’s faculty page.
Also listed in PubMed.