Hewitt Lab
The Hewitt lab investigates mechanisms that guide physiological and regenerative blood formation. They are especially absorbed with understanding how gene regulation is controlled during the differentiation of all the varied cell types that exist in the blood system, the role of the GATA family of transcription factors in development, and diseases that arise from deregulated transcription factor activities including chronic anemias and leukemias.
Major ongoing projects in the lab include:
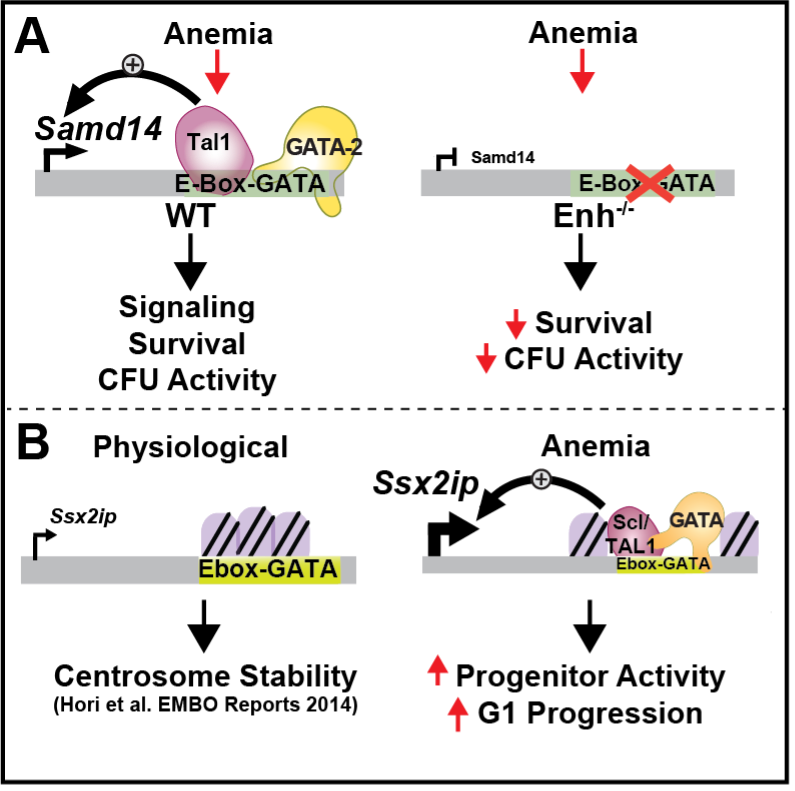
Fig 1A and Fig 1B
1. Determining cis-regulatory control mechanisms in erythroid regeneration. Anemia-activated cis-elements are controlled by GATA factors. During red blood cell regeneration, a Kit+, GATA2-expressing population of self-renewing erythroid-restricted cells rapidly expand and differentiate. The GATA family of transcription factors function to activate/repress complex genetic circuits. A GATA-occupied cis-element at the Samd14 locus (S14E) was required for survival in anemia (Fig 1A). A GATA2-Samd14-Kit axis regulates erythroid regeneration, and SAMD14 dysregulation may have pathogenic consequences. Using the sequence/molecular properties of the S14E anemia-activated enhancer as a guide, we also described similar anemia-dependent enhancer activities at the Ssx2ip locus, encoding a gene upregulated in leukemia and predicted to control centromere assembly (Fig 1B).
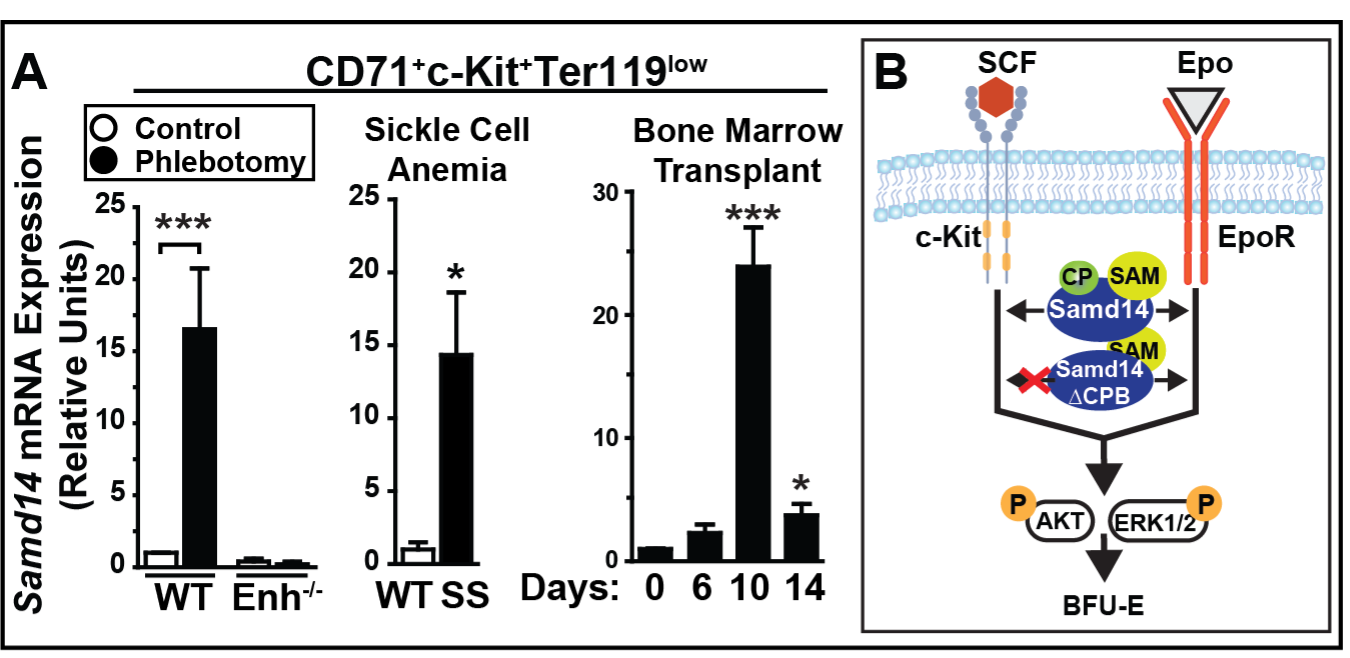
Fig 2A and Fig 2B
2. The coordination of cell signaling to regulate transcriptional activation and progenitor activities in anemia and leukemia. The Samd14 gene and protein are upregulated in several acute/chronic anemia contexts (Fig 2A), but its precise functional roles are unknown. We are investigating how Samd14 coordinates cell signaling during the process of anemia recovery through oncogenic cell signaling pathways that drive progenitor activity (Fig 2B).
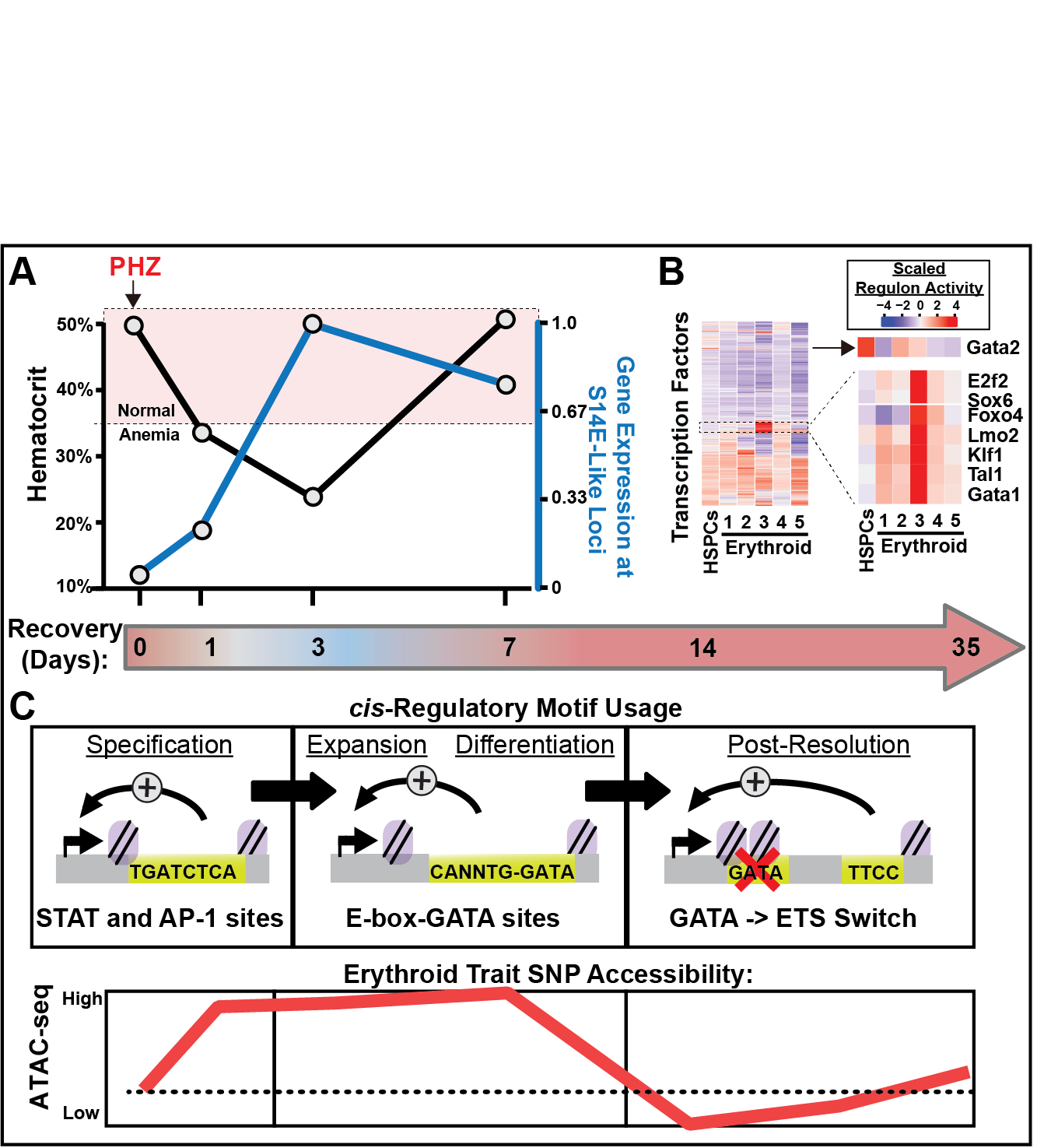
Fig 3A, Fig 3B, and Fig 3C
3. GATA factor mechanisms in erythroid regeneration. Acute regeneration of hematopoietic cell types induces big changes to gene transcription (Fig 3A). Sub-types of erythroid precursors have unique predicted TF activities that increase in anemia stress (Fig 3B). Dozens of genes associated with GATA-occupied enhancers resembling S14E are upregulated in erythroid progenitor cells post-anemia. Molecularly-similar enhancers represent a functional subgroup of cis-elements in a broader erythroid gene network, some of which appear to have polymorphisms linked to erythroid traits in humans (Fig 3C). Anemia stress-dependent transcriptional mechanisms can be used to develop new clinical strategies to regenerate erythroid cells and/or to increase the regenerative capacity of existing populations of human HSPCs, often associated with natural variation in human polymorphisms that may be useful for development of personalized medicine approaches to treating hematologic diseases such as anemia and leukemia.
Principal Investigator
Kyle J. Hewitt, PhD
Associate Professor, Department of Genetics, Cell Biology, and Anatomy
Co-Director, Molecular Genetics & Cell Biology PhD Program
Director, Single Cell and Spatial Transcriptomics Core
