Rizzino Lab
The major focus of this laboratory has been to understand the molecular mechanisms by which the stem cell transcription factor, SOX2, regulates the self-renewal of stem cells and the growth of human tumor cells.
We are focused on SOX2, because it is expressed in at least 20 types of human cancer, including their cancer stem cell populations.
Director
Angie Rizzino, PhD
Professor, Eppley Institute for Research in Cancer and Allied Diseases
Research focus: Transcription regulation; stem cells
Lab Findings
Our initial focus, starting in mid-1990s, was on the transcriptional activity of SOX2 in embryonic tumor cells. More recently, we have directed our attention to the surprising finding that the effect of SOX2 on the proliferation of tumor cells, as well as that of normal cells, is highly dependent on the dosage of SOX2.
Importantly, we discovered that SOX2 functions as a biphasic molecular rheostat in tumor cells. More specifically, we determined that both elevating SOX2 and knocking down SOX2 in tumor cells lead to dramatic reductions in tumor growth.
Consistent with our findings, there is now a significant body of evidence directly linking SOX2 to tumor cell quiescence and dormancy. This is very important, because dormant tumor cells play a critical role in tumor recurrence and drug resistance, both of which are major clinical challenges. To meet these challenges, we are focusing on the molecular mechanisms by which elevated levels of SOX2 induce growth arrest.
With this in mind, our long-term goal is to identify strategies to eradicate dormant tumor cells by identifying their vulnerabilities.
Increasing SOX2 Levels in SOX2-Expressing Tumor Cells Reduces Their ProliferationIn 2008, we demonstrated that elevating SOX2 by as little as 2-fold in embryonic stem cells disrupts their self-renewal and rapidly triggers differentiation. This is due, at least in part, to transcriptional repression of multiple stem cell transcription factors (e.g. Oct4 and Nanog).
Our work in embryonic stem cells led us to focus heavily on the roles of SOX2 in multiple cancers, including pancreatic ductal adenocarcinoma (PDAC), prostate cancer (PCa), glioblastoma, and medulloblastoma. Remarkably, we determined that SOX2 levels in each of these cancers are optimized to maximize tumor growth – SOX2 levels that are too high or too low reduce tumor growth.
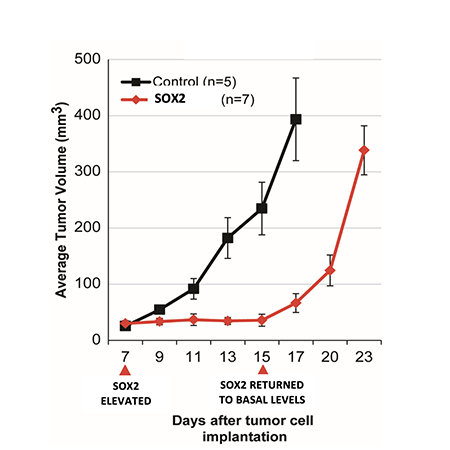
Fig. 1, Metz et al Cancers, 2022: Tumor growth is completely halted when SOX2 is elevated in vivo, but rapidly resumes when SOX2 is returned to its basal levels.
In the case of elevated SOX2, tumor growth is completely halted when SOX2 is elevated in vivo, but rapidly resumes when SOX2 is returned to its basal levels (Figure 1, Metz et al Cancers, 2022). Thus, elevated levels of SOX2 in tumor cells can induce a reversible state of tumor growth arrest (tumor dormancy).
Importantly, our work with these cancers indicate that this is a defining feature of SOX2. This surprising property of SOX2 is fully consistent with the wide array of molecular mechanisms used to tightly control the expression, subcellular localization, and activity of SOX2. These mechanisms include regulation at both the transcriptional level, by the action of multiple distal enhancers, and at the post-transcriptional level, by more than 16 microRNAs and by more than a dozen post-translational modifications.
SOX2 Regulates Tumor Cell Proliferation Through a SOX2:MYC Axis
Recent studies in this laboratory discovered that elevating SOX2 strongly downregulates MYC and MYC target genes. In the case of MYC, we have shown that it is downregulated at both the RNA and protein level in cells representing at least four tumor types.
Moreover, using nuclear run-off assays, we determined that MYC is downregulated at the transcriptional level. Currently, we are focusing on the molecular mechanisms by which elevated SOX2 downregulates MYC at the transcriptional level.
Effects of Stable Overexpression of SOX2 and Expression from an Inducible Promoter Generate Different Outcomes
Over the past dozen years, there have been many studies reporting that increasing the expression of SOX2 in a wide range of SOX2-positive tumor cells [e.g. glioblastoma, prostate cancer, and pancreatic ductal adenocarcinoma (PDAC)] enhances their proliferation both in vitro and in vivo. Our recent studies argue strongly that these findings need to be reevaluated.
In each of the earlier studies, tumor cells were engineered to stably overexpress SOX2. Remarkably, we have observed completely different results when tumor cells are first engineered to express SOX2 from a doxycycline-inducible promoter and later doxycycline is added to the culture medium to elevate SOX2.
Importantly, our studies demonstrate that elevating SOX2 with the aid of an inducible promoter in >20 tumor cell lines, which represent eight different tumor types, reduces proliferation of each tumor cell type.
The contrasting results obtained when SOX2 is stably overexpressed versus our inducible overexpression studies are due to a fundamental difference in experimental design. Our tumor cell lines engineered for inducible overexpression of SOX2 are generated via drug selection of lentiviral-transduced cells, which occurs at frequencies greater than 70%.
In our studies, SOX2 levels are only elevated after the transduced cells have been established and the non-transduced cells have been eliminated by drug selection (puromycin). In direct contrast, cell lines engineered to stably overexpress SOX2 are subjected to drug selection when SOX2 levels are ectopically elevated from the beginning.
As a result, any cells that are unable to proliferate or grow more slowly due to elevated levels of SOX2 will be lost during the drug selection period as the faster proliferating cells expand. Consequently, the cells present in these drug-selected populations represent only a subpopulation of the parental cells. Our studies indicate that this is an exceedingly small subpopulation.
SOX2 is Part of a Highly Integrated Transcriptional Network
As part of our efforts to understand why small changes in the levels of SOX2 influence the self-renewal of tumor cells, we conducted unbiased proteomic screens to identify the SOX2-interactome in multiple cell types, including embryonic cells, glioblastoma and medulloblastoma cells.
These studies led to three important findings:
- SOX2 interacts with a wide range of proteins involved in transcription, DNA repair/replication, and signal transduction.
- SOX2 is part of a highly interdependent protein:protein interaction network that integrates cellular function at multiple levels.
- Elevating SOX2 in tumor cells dramatically alters their SOX2-interactome.