Current Projects
Alcohol-associated liver disease
Lipids serve as a critical source for cellular energy, but in many metabolic diseases, excessive fat accumulates at a rate that is higher than the cell’s capacity to degrade. This is particularly important in liver cells (hepatocytes), which serve as a central site for the body’s metabolism and process nutrients, minerals, and toxins from the blood. Excessive accumulation of lipid in hepatocytes is a key feature of fatty liver disease (i.e. hepatic steatosis) and is a major health concern affecting 25% of the world’s population. Causes of fatty liver include, but are not limited to, obesity, alcoholism, viral infection, and/or specific genetic mutations. If left untreated, fatty liver leads to increasing amounts of inflammation and irreversible liver damage in the forms of fibrosis, cirrhosis, or highly lethal forms of liver cancer. Invasive and costly liver transplants are currently the best treatment for advanced liver disease. Because of this, there is an urgent need to understand how cells metabolize lipids to reverse the progression of these end-stage liver diseases. The central focus of my lab is to discover new cellular mechanisms of lipid metabolism, with the hope that this knowledge would translate to effective therapies for metabolic diseases.
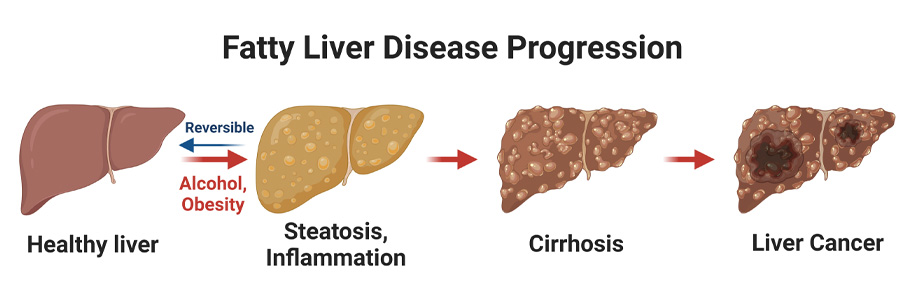
Early stages of fatty liver disease (steatosis, steatohepatitis) are induced by alcohol, obesity, and/or other factors. These early stages are reversible, but later stages of disease (cirrhosis, cancer) are irreversible and life-threatening, often requiring liver transplant. Created with BioRender.com.
Lipid droplet trafficking mechanisms
Lipids are stored within cells by specialized organelles known as lipid droplets (LDs). Lipid droplets are formed at the endoplasmic reticulum, and sequester fats that would otherwise be toxic in the cytoplasm. Multiple organelles physically interact with LDs including mitochondria, endoplasmic reticulum, peroxisomes, endosomes, and lysosomes to regulate cellular energy. LDs have a unique proteome that dictates intracellular trafficking, LD-LD fusion, and recruitment of enzymes that facilitate the breakdown of these organelles. Breakdown of LDs requires lipases (lipid-catabolizing enzymes) that are recruited directly to the LD surface from the cytoplasm, but LDs are also targeted by specific mechanisms of autophagy, a “self-eating” process important for cellular energy and survival during times of low nutrient availability. Many interesting and important questions remain about these distinct LD-catabolic processes. For example, how are LDs specifically targeted by autophagic vesicles, and how is this controlled by nutrient availability? What forms of autophagy are most important? How are cytosolic lipases controlled by various hormone receptor signaling pathways, and how does this impact LD autophagy? How is lipid metabolism affected by various metabolic diseases? Understanding these cellular mechanisms will aid in the development of new therapies to combat fatty liver and its downstream consequences to human health.